
by Jack Norris, RD, LD
Contents
- Essential Information
- Dietary Reference Intakes for ALA
- Plant Sources of ALA
- Additional Tips
- Research on Omega-3 Fatty Acids in Plant-Based Diets
- Background on Omega-3s
- Essential Fatty Acid Intakes of Vegans
- Long-chain Omega-3 Fatty Acid Blood Levels of Vegetarians
- Conversion of ALA to EPA and DHA
- DHA Supplementation in Vegetarians
- Omega-3 Recommendations for Vegans
- Vegetarian Pregnancy, Nursing, and Infants
- Omega-3s and Chronic Disease
- Appendix A: Biomarkers of DHA Status
- Appendix B: Evolutionary Arguments for a Dietary Requirement for DHA
- Appendix C: Stereodonic Acid
- Appendix D: Salvador, 2019
- Bibliography
Essential Information
Omega-3 fats are important for the long-term health of the heart and brain but are found in a limited number of plant foods. Walnuts, canola oil, flaxseeds and flaxseed oil, chia seeds, hemp seeds, and perilla oil are high in omega-3s.
Chia Seed Pudding Recipe
A delicious way to get your daily omega-3s is from chia seed pudding, which you can eat for breakfast or as a dessert.

Ingredients
- 1-3/4 cups of unsweetened non-dairy milk (or sweetened non-dairy milk and avoid the sweetener ingredient below)
- 1 to 2 tablespoons of sweetener (for example, sugar or maple syrup)
- 1/2 cup of chia seeds
- 1/2 to 1 teaspoon of vanilla extract (optional)
Instructions
- In a bowl, whisk together the ingredients.
- Chill for a few hours and stir before eating.
- Even better served with toppings, such as fruit, peanut butter, or chocolate chips.
- Keep refrigerated.
Another option is to keep a jar of hemp or ground flaxseeds in the refrigerator to sprinkle them on meals throughout the day—they’re easy to incorporate into anything you’re eating.
Dietary Reference Intakes for ALA
The table below lists the Dietary Reference Intakes (DRI) for the essential omega-3 fat, alpha-linolenic acid (ALA).
| Dietary Reference Intakes for ALA | ||
|---|---|---|
| Age | Male mg/day |
Female mg/day |
| 1-12 monthsA | 500 | 500 |
| 1-3 | 700 | 700 |
| 4-8 | 900 | 900 |
| 9-13 | 1,200 | 1,000 |
| 14+ | 1,600 | 1,100 |
| Pregnancy | 1,400 | |
| Breastfeeding | 1,300 | |
| AAI for infants less than 1 year old is for total omega-3s (ALA + EPA + DHA). The Institute of Medicine doesn’t give specific recommendations for any individual omega-3. | ||
Plant Sources of ALA
| ALA Amounts in Plant Foods | |||
|---|---|---|---|
| Food | Size | ALA mg | Source |
| Camelina oil | 1/2 teaspoon | 700 | Budin, 1995 |
| Canola oil | 1 teaspoon | 411 | USDA |
| Chia seeds | 1 teaspoon | 713 | Norris, 2024 |
| Flaxseed oil | 1/4 teaspoon | 605 | USDA |
| Flaxseeds, ground | 3 g (~1 teaspoon) | 580 | USDA |
| Hemp seed oil, refined | 1 teaspoon | 750 | Mikulcová, 2017 |
| Hemp seed oil, unrefined | 1 teaspoon | 900 | Mikulcová, 2017 |
| Perilla oil | 1/4 teaspoon | 625 | Gwari, 2014; Longvah, 2000 |
| Soybeans, cooked | 1/2 cup | 515 | USDAB |
| Soy oil | 1 teaspoon | 311 | USDA |
| Tempeh | 1 cup | 412 | USDAB |
| Tofu, firm | 1 cup | 420 | USDAB |
| Walnuts, English (light brown)A | 3 halves (6 g) | 551 | USDAB |
| AAlways grind nuts and mix with food for 1 to 4 year olds to avoid choking. BUSDA doesn’t distinguish between PUFA 18:3 ALA and other PUFA 18:3 fatty acids for this food. | |||
Additional Tips
Whether vegans need to do more than meet the DRI for ALA for a normal omega-3 status is controversial (and discussed in great depth below).
To be extra cautious, vegans can take one of these additional steps:
- Consume an additional 2,000 mg of ALA per day using the foods in the table above.
- Take a supplement of 200-300 mg of DHA per day.
Your DHA supplement can contain EPA, but it’s not necessary to contain EPA if you’re meeting the DRI for ALA. We don’t recommend or have opinions on any specific brands of DHA supplements.
Too much omega-3 can result in bleeding and bruising. If you bleed or bruise easily, consult a health professional before significantly increasing your omega-3 intake.
See below for our recommendations for pregnancy, nursing, and infants.
Flax
- If flaxseeds are not ground, they will not be digested (Austria, 2008). They can be ground in a blender (works best with a large amount) or coffee grinder, and then stored in the freezer. Ground flaxseeds can be sprinkled on cereal or used in baked goods.
- There is some evidence that people 45 and older do not absorb the oil from ground flaxseeds as well as from flaxseed oil (Patenaude, 2009). The one study indicating this was only for four weeks and used 6 g of ALA per day. With smaller amounts and for longer periods, the difference might be negligible, but that hasn’t been tested.
- Cooking flaxseed oil damages the ALA, but it can be put on warm food such as toast.
- Flaxseed oil should be kept in the refrigerator.
- Flaxseed oil doesn’t taste very good. Some people use cinnamon-flavored oil, tablets, or put it on toast or salad to disguise the taste.
Research on Omega-3 Fatty Acids in Plant-Based Diets
There are two questions regarding vegetarians and omega-3s: Do vegetarians have negative health consequences from not eating fish and should vegetarians supplement with the omega-3s typically found in fish (EPA and DHA)? Although vegetarians and vegans have been shown in many studies to have lower blood levels of EPA and DHA than fish-eaters, the quick answer to these questions is that there isn’t sufficient evidence to conclude that the lower levels have negative health consequences.
The rest of this article discusses the arguments and research surrounding this issue.
Background on Omega-3s
For our purposes, there are four important omega-3 fatty acids:
- alpha-linolenic acid (ALA) • Short-chain omega-3 fatty acid (C18:3n-3). Found in small amounts in animal flesh, in very small amounts in a variety of plant products, and in relatively large amounts in soy, walnuts, canola oil, flaxseeds and their oil, hempseed oil, camelina oil, perilla oil, and chia seeds. The human body cannot make its own ALA; it must be obtained through the diet.
- eicosapentaenoic acid (EPA) • Long-chain omega-3 fatty acid (C20:5n-3). Found mostly in fatty fish, in small amounts in eggs, and in very small amounts in seaweed that can be concentrated into supplements. Some EPA is converted into series 3 eicosanoids which can reduce blood clotting, inflammation, blood pressure, and cholesterol. The human body can produce EPA from ALA and possibly from DHA.
- docosapentaenoic acid (DPA) • Long-chain fatty acid. There is both an omega-3 (C22:5n-3) and omega-6 (C22:5n-6) version of DPA. The omega-3 version is an intermediary between EPA and DHA.
- docosahexaenoic acid (DHA) • Long-chain omega-3 fatty acid (C22:6n-3). Found mostly in fatty fish, in small amounts in eggs, and in very small amounts in seaweed that can be concentrated into supplements. DHA is a major component of the gray matter of the brain, and also found in the heart, retina, testis, sperm, and cell membranes. The body can convert EPA into DHA.
The chart below shows the conversion pathways for the omega-3 and omega-6 fatty acids. In the chart, D6D is the enzyme that converts ALA and LA into other fats.
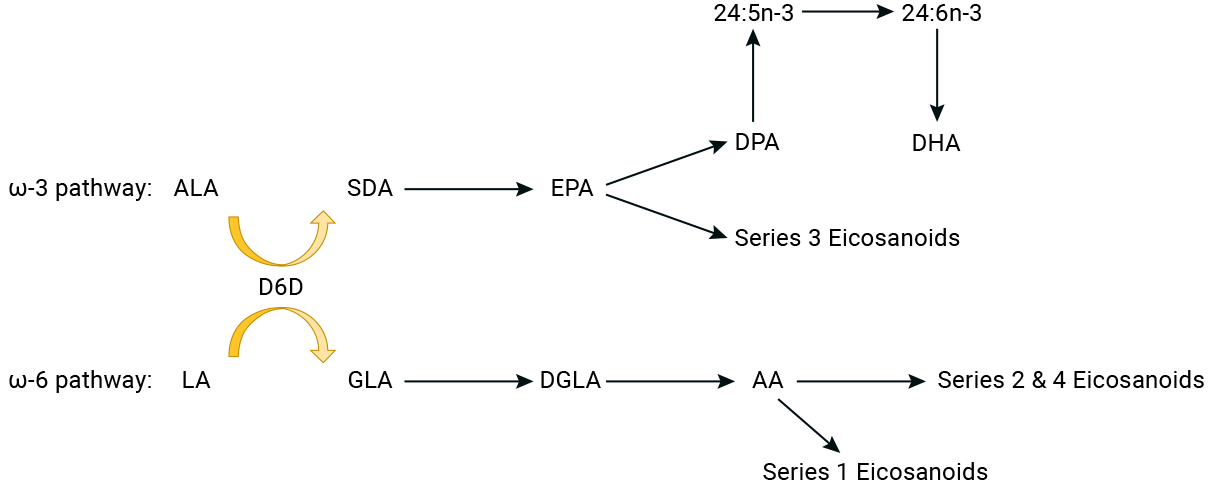
See the video below for an excellent overview of omega-3 fatty acids from omega-3 researcher Dr. Richard Bazinet of the University of Toronto (2021).
Essential Fatty Acid Intakes of Vegans
The Institute of Medicine considers there to be a dietary requirement for two fatty acids for people age 1 year and older, alpha-linolenic acid (ALA) and linoleic acid (LA). However, Burdge (2022) reports that an overt deficiency of ALA has never been fully isolated from LA and fat-soluble vitamin deficiencies in adult humans in order to clearly demonstrate that ALA is an essential fatty acid.
The table below shows the weighted averages of studies measuring vegan ALA intakes. Calculations and citations are in our ALA Intakes spreadsheet.
The World Health Organization and Food and Agriculture Organization (2010) recommend an LA intake between 2.5% to 9% of calories, saying that the lower number prevents deficiency and the higher end of the range reduces the risk for heart disease. Although vegans who don’t ensure sources of ALA tend to have a high ratio of omega-6 to omega-3 fats, their percentage of calories as LA has been shown to be 5.1% (Pinto, 2017, United Kingdom), 7.3% (Allès, 2017, France), 8.5% (Kornsteiner, 2008, Austria), and 9.3% (Rizzo, 2013, USA), well within the range recommended by the WHO.
Long-chain Omega-3 Fatty Acid Blood Levels of Vegetarians
Summary: The differences in long-chain omega-3 blood levels between vegans, lacto-ovo-vegetarians, and omnivores aren’t obviously physiologically significant, especially with regard to omnivores who don’t regularly eat fish. Red blood cell DHA of vegetarians and vegans is roughly 72-75% of that of omnivores, but it’s not clear if this has clinical significance.
There is no standardized method for measuring omega-3 fatty acids: no one knows what levels of fatty acids in any given medium represent a deficient, healthy, or optimal level. It could even be that blood levels of fatty acids have little bearing on omega-3 fatty acid status. The purpose of this section is to determine whether vegans do indeed have lower blood levels of long-chain omega-3 fatty acids than omnivores. Early studies found that vegans have lower EPA and DHA blood levels, but these studies were conducted on very few people; more recent studies haven’t shown nearly the difference.
The way omega-3s are measured among these studies varies considerably. Fatty acids can be measured in various components of plasma such as phospholipids, triglycerides, or cholesterol esters. Fatty acids may also be measured in the adipose tissue, platelets, or red blood cells. Because red blood cells have a lifespan of 120 days, red blood cell fatty acids might be a more accurate long-term representation of omega-3 status.
As of early 2022, we’ve tracked 27 studies measuring the blood levels of omega-3 fatty acids in vegetarians. We list these studies and their measurements in the Cross-sectional tab of our spreadsheet, Omega-3s Part 2: Research. In the plasma, omega-3s are usually measured as a percentage of total fatty acids, but Welch et al. (2010) measured omega-3s as a concentration in plasma and Rosell et al. (2005) provided the data to calculate a concentration. Concentrations might be a more accurate reflection of the body’s omega-3 stores since they represent an absolute rather than a relative amount.
DPA is a long-chain omega-3 fatty acid that is an intermediary between EPA and DHA. We emphasize studies that included DPA in their measurements because DPA represents a significant fraction of long-chain omega-3s that vegans have converted from ALA and which can potentially be converted to DHA.
The graph below plots all measurements that compared total long-chain omega-3 levels (EPA+DPA+DHA) of vegetarians or vegans to omnivores. It includes measurements of percentages and concentrations for each medium. While there’s considerable overlap between diet groups, individual studies generally find that omnivores have higher levels of long-chain omega-3s than vegans with the differences being statistically significant.
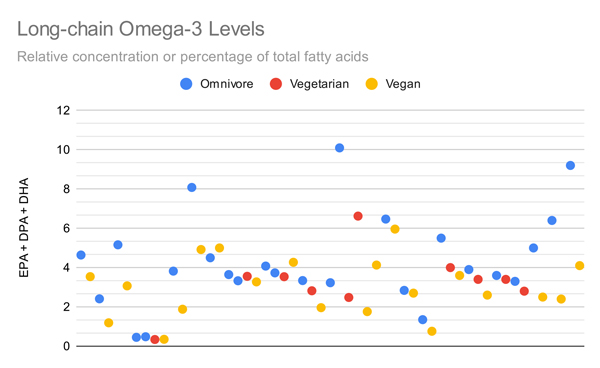
The graphs below compare only the EPA or DHA levels of vegans and vegetarians in all studies that measured EPA or DHA.
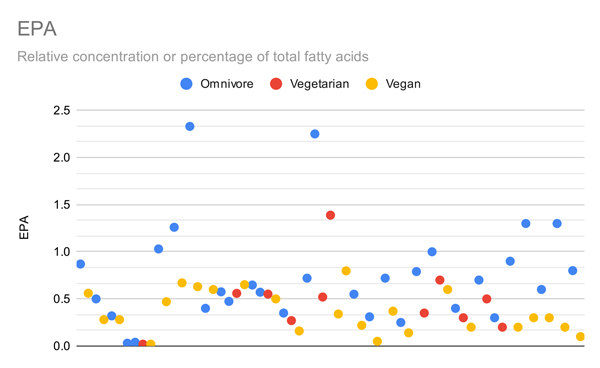
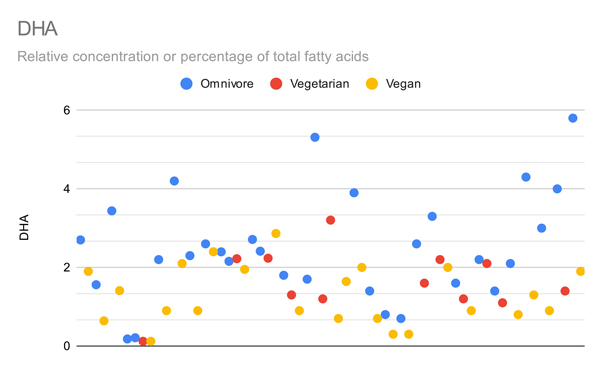
Arguably the most important metric is red blood cell omega-3s, shown in the graph below.
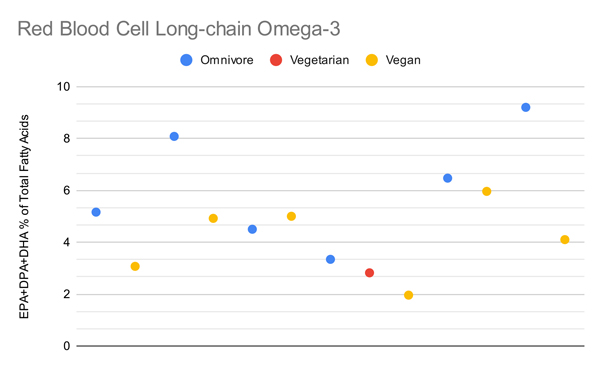
It’s hard to conclude much regarding vegan long-chain omega-3 levels from these studies given that the measurements aren’t standardized, aren’t well understood, and contain significant overlap. Arguably a more accurate way to assess this data is to weight the comparisons of vegetarians both proportional to the omnivores in the same studies and proportional to how many people were in each diet group while limiting the measurements to one per population studied.
In order to get the most accurate picture of how long-chain omega-3 blood levels of vegans compare to those of omnivores, we decided to calibrate the measurements by creating a ratio of the levels of vegans to those of omnivores rather than using an absolute amount. We did this by simply dividing the vegan level by the omnivore level.
For example, the study by Kornsteiner et al. found an EPA+DPA+DHA percentage of total fatty acids in red blood cells of 1.96% for vegans and 3.34% for omnivores. The study by Li et al. found an EPA+DPA+DHA percentage of total fatty acids in plasma of 3.6% for vegans and 5.5% for omnivores. We don’t know if we can compare the percentage of fatty acids in red blood cells to the fatty acids in plasma, but we can compare the ratio of vegan to omnivore long-chain omega-3s in both studies, which was .59 in Kornsteiner et al. and .65 in Li et al. We can then multiply these two ratios by the number of vegans in their respective study, divide by the total number of vegans in both studies, and get a weighted average of the ratio of vegan to omnivore long-chain omega-3s across both studies. By weighting all of the studies in this way, we can obtain the most accurate picture of how blood levels of long-chain omega-3 fatty acids compare for vegans and omnivores.
Most studies measured omega-3s as a percentage of total fatty acids; to be as consistent as possible, we weighted the percentage of total fatty acids rather than the concentration for studies that measured both. For studies with multiple measurements, we chose in this order: red blood cells, plasma, platelets, and adipose tissue.
The table below shows the weighted proportions of omega-3s for vegetarians and vegans compared to omnivores for all studies and for red blood cell (RBC) measurements only. Calculations and citations are in the Cross-sectional tab of our spreadsheet, Omega-3s Part 2: Research.
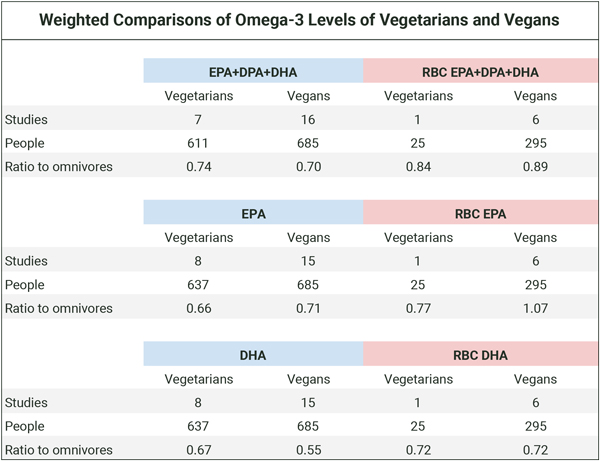
Based on the table above, vegans generally have lower blood levels of long-chain omega-3s than omnivores. Since plasma levels of omega-3s are at least in part a representation of dietary fatty acids, as distinct from representing only the body’s ability to convert dietary short-chain to long-chain omega-3s, it’s not surprising that people who have an intake of long-chain omega-3s have higher blood levels.
Vegetarians vs. Fish-Eaters
Among people who don’t supplement with long-chain omega-3s, regular fish-eaters will be the only dietary group with a significant source of long-chain omega-3s. According to the USDA nutrient database, a medium egg contains about 2 mg of EPA and 16 mg of DHA. That provides lacto-ovo-vegetarians with very small amounts of dietary EPA and DHA.
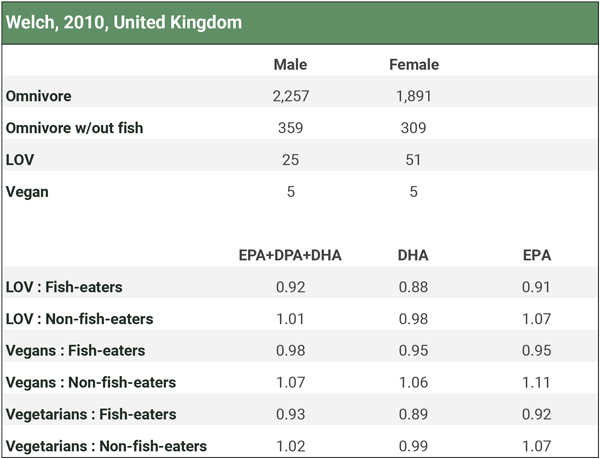
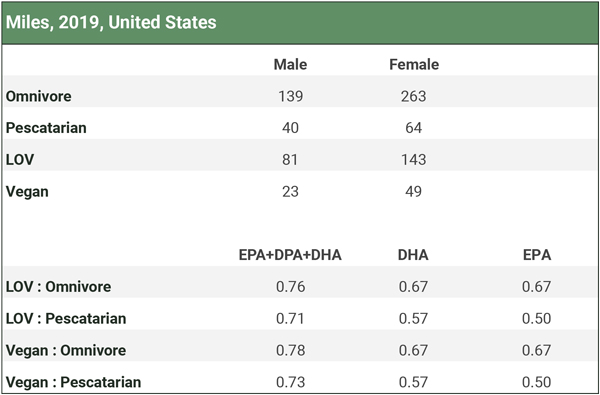
There are two studies that measured omega-3 levels among fish-eaters (Welch, 2010; Miles, 2019), but neither measured it in red blood cells. We analyze these studies in the Fish-eaters tab of our spreadsheet Omega-3s Part 2: Research and summarize the results in the three charts below. Participants in the studies didn’t use long-chain omega-3 supplements.
Welch et al. (2010) measured omega-3 plasma concentrations and separated omnivores into groups who did and did not eat fish. There were only 10 vegans.
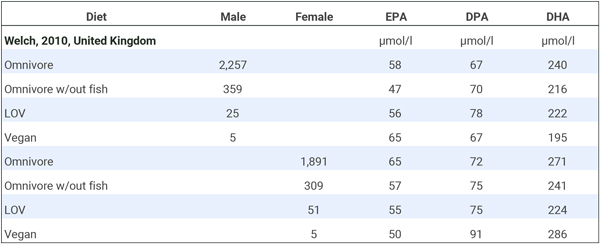
We combined the male and female long-chain omega-3 plasma concentrations to determine how vegans compared to both fish-eating and non-fish-eating omnivores. Because there were so few vegans, we also combined the lacto-ovo-vegetarians (LOV) with the vegans for a “vegetarian” category. The table below shows that lacto-ovo-vegetarians, vegans, or the combined group had levels slightly below fish-eaters and either similar or higher levels than non-fish-eaters.
Miles et al. (2019) compared the percentage of omega-3 fatty acids in the adipose tissue of pescatarians to other dietary groups, as shown in the table below. Vegetarians and vegans had lower levels than fish-eaters and even somewhat lower levels than omnivores. Although vegans had substantially lower levels than fish-eaters in this study, it’s not clear what the percentages of fatty acids in adipose tissue represent; possibly nothing of clinical significance.
Fatty Acid Levels of Older vs. Younger Vegans
It’s normally thought that people have a harder time converting ALA to EPA and DHA as they age. In contrast, Sarter et al. (2015) found that 69 vegans aged 60 to 85 had EPA+DHA levels of about 4.0% compared to about 3.6% for 97 vegans aged 20 to 59 (p for trend = 0.009).
Impacts of Lower EPA and DHA on Vegetarians
A possible benefit of long-chain omega-3 fatty acids, especially EPA, is reduced blood clotting which protects against heart attacks. There have been some differences noted in blood clotting between vegetarians and omnivores.
Mezzano et al. (1999, Chile), found that vegetarians had significantly more platelets (242,000 per ul) than non-vegetarians (211,000 per ul) and a shorter bleeding time (4.5 vs. 7.3 min), which could increase the risk of a cardiovascular event. In a follow-up study, Mezzano et al. (2000, Chile) gave vegetarians 700 mg EPA and 700 mg DHA for 8 weeks. EPA went from .2 to 1.8% and DHA went from 1.1 to 3.0%. Some clotting factors changed, but bleeding time remained lower at 5-1/2 minutes.
Sanders and Roshani (1992, United Kingdom) found that one of eight platelet aggregation parameters in vegan men, but not women, was different from the non-vegetarians. Bleeding times were similar.
Pinto et al. (2017, United Kingdom) compared heart rate variability between a group of 23 adult vegans and 24 omnivores. Low heart rate variability reflects a reduced capacity for the heart to respond to the body’s physiological demands and is linked to an increased risk for heart disease. As expected, the vegans had lower concentrations of DHA and EPA in both red blood cells and plasma. While vegans had a higher heart rate variability over a 24-hour period, their daytime heart rate variability was lower, and their heart rate was greater. The clinical significance of these findings aren’t clear.
Thus, of three studies that looked at cardiovascular markers, the results are mixed.
In terms of cognition, in their study of British mortality, Appleby et al. (2002) found vegetarians to have a barely statistically significant, higher risk of death from mental and neurological diseases (DRR 2.21, CI 1.02–4.78). In contrast, a more recent report from EPIC-Oxford (Appleby, 2016) found that vegetarian deaths from mental and behavioral disorders were not statistically different from non-vegetarians (HR 1.22, CI 0.78–1.91). And a report from the Adventist Health Study-2 (Orlich, 2013, USA) found no difference in mortality from neurologic diseases between vegetarians and non-vegetarians (HR 0.93, CI 0.67-1.29); pescatarians and semi-vegetarians were included in their vegetarian category so the results can’t be extrapolated to vegetarians who don’t eat fish.
Conversion of ALA to EPA and DHA
Measurements of the percentage of total fatty acids as EPA and DHA in the blood are generally considered a marker of omega-3 status. This assumes that higher percentages of total fatty acids in the blood reflect higher and more optimal amounts in the tissues that utilize omega-3s. It also assumes that when blood percentages change due to changes in dietary intake, levels in tissues respond similarly.
In this section, we examine these assumptions. Evidence of omega-3 conversion enzymes in tissues and down-regulation of omega-3 conversion in response to dietary omega-3s suggest that the body can regulate the conversion of omega-3 fatty acids in tissues independent of the percentage in the blood.
There’s evidence that high intakes of EPA and DHA will increase their percentages in both blood and tissues, but it’s not clear if higher percentages are necessary for optimal health. We assess the evidence in our sections Impacts of Lower EPA and DHA on Vegetarians and Omega-3s and Chronic Disease.
ALA Supplementation Results in Little Increase in Blood DHA
Our ALA Trials spreadsheet lists a handful of clinical trials, including all of the trials with vegetarians of which we’re aware, investigating whether increasing dietary ALA subsequently increases the percentage of long-chain omega-3s in the blood. The changes in total fatty acids as long-chain omega-3s show a wide variation with no clear pattern; some even found a decrease in DHA. On average, EPA+DPA+DHA increased by 43.5% while DHA only increased by 4.6%.
It’s safe to say that supplementing with ALA is unlikely to substantially increase the blood percentage of fatty acids as DHA in most adults.
EPA and DHA Correlate between Plasma and the Heart but not the Brain
Summary: Based on limited, mostly cross-sectional data, there appears to be a robust correlation between the blood and tissue percentages of EPA+DHA in the human heart but not the brain or sperm.
Studies of ALA supplementation result in very little increase of DHA in the blood, but how much evidence is there to suggest that this reflects the body’s inability to convert ALA to DHA for tissue utilization?
A basic question is, without any dietary changes, how much do blood levels of omega-3 fatty acids typically correlate with tissue levels? It’s difficult to study the omega-3 content of tissues in living humans. In our spreadsheet, Tissue Correlations, we list the correlations between blood and tissue percentages of omega-3s in both humans and animals. A summary of the results follows.
Harris et al. (2004) measured the correlation between the percentage of EPA+DHA in red blood cells and the percentage of EPA+DHA in the hearts of 20 heart transplantation patients having routine heart biopsies, 13 of whom were considered to be high consumers of EPA and DHA; they found a statistically significant, strong correlation (R = 0.82, P ≤ 0.0001).
Harris et al. (2004) also performed an intervention: Heart transplantation patients (n=25) with low EPA+DHA intakes were provided 1,000 mg of EPA+DHA for 6 months. These patients had weaker correlations between red blood cell and heart EPA+DHA at baseline (R = 0.47, P = 0.031). Post-intervention measurements showed that EPA+DHA percentages increased in plasma, red blood cells, heart, and cheek tissue; the correlation between red blood cell and heart EPA+DHA remained the same (R = 0.47, P = 0.06).
Metcalf et al. (2007) placed a series of patients on ALA (5.8 g per day) or EPA+DHA (6.3 g, ~50% each) for a number of weeks based on their heart surgery schedule. While they didn’t provide a correlation parameter between red blood cell and heart omega-3 fatty acid percentages, the percentages of the two mediums were fairly similar and differed from the control group in similar amounts post-treatment (see our spreadsheet ALA Trials).
Cunnane et al. (2012) performed autopsies on cognitively normal people and found a correlation between percentages of DHA in plasma phosphatidylethanolamine and the angular gyrus region of the brain DHA (R = 0.77, P ≤ 0.005). However, they failed to find correlations between DHA and other regions or in cognitively impaired people stating, “No significant correlations were observed for DHA (% or mg/g) or any other fatty acids in the other brain regions or in the [Alzheimer’s disease] and [mildly cognitively impaired] groups (data not shown).”
Carver et al. (2001) performed autopsies on 58 people and found a negative correlation between the DHA percentage in red blood cells and the cerebral cortex of people aged >18 years; it’s likely this correlation doesn’t achieve statistical significance after a Bonferroni correction for the large number of correlations tested.
Chamorro et al. (2020) measured the fatty acid percentages of young men, comparing vegans (n=34) and omnivores (n=33). They didn’t test for a correlation between the percentage of omega-3s in plasma or red blood cells and sperm. The ratio of the percentage of EPA in sperm to that in plasma and red blood cells was similar at 0.54 for each, but the ratios for DPA and DHA were not. See the table below.
There’s much more data from animals than humans. Our spreadsheet, Tissue Correlations, lists 24 correlations between blood and tissue percentages of EPA+DHA among rats, pigs, and mice. The strength of the correlations varies considerably with some being negative.
There’s one other study on animals worth mentioning. Talahalli et al. (2010) fed two groups of rats a reasonable amount of ALA (2.5% and 5.0% of calories). After 60 days, the percentage of fatty acids as DHA in the brain of the rats fed 2.5% and 5.0% ALA was, respectively, 9.4% and 10.4% compared to 8.3% in the control group (see the table, Talahalli 2010). This suggests that ALA supplementation increased the amount of DHA in their brains.
One significant caveat for comparing the conversion of omega-3s in rats, pigs, and mice to humans is that rats, pigs, and mice normally don’t have a dietary source of EPA or DHA and, therefore, would normally rely entirely on the conversion from ALA for any EPA or DHA.
While plasma and red blood cell percentages of long-chain omega-3 fatty acids sometimes correlate with tissues, they don’t do so consistently. It might be possible to develop a mathematical model that can account for the large number of variables that impact the correlations but it seems unlikely that a model will be developed that gives us confidence in predicting the omega-3 tissue status of vegetarians.
In Appendix A, I discuss a review paper, Biomarkers of DHA status, arguing that blood percentages are adequate markers of DHA status.
Tissues Contain Enzymes that Convert Omega-3s
Two critical enzymes, delta-5 desaturase and delta-6 desaturase, convert short-chain omega-3 and omega-6 fatty acids into long-chain versions.
Previously, the liver was considered the primary site of EPA and DHA production for peripheral tissue utilization, but studies by Cho et al. (1999a and 1999b) found substantial amounts of mRNA for the delta-5 and delta-6 desaturase enzymes in many tissues of human cadavers.
Cho et al. (1999a) found that delta-5 desaturase mRNA was greatest in the human liver, but that the heart, brain, and lung also contained substantial amounts. They found low but detectable levels in the placenta, skeletal muscle, kidney, and pancreas. Cho et al. (1999b) found that the amount of delta-6 desaturase mRNA in the human liver was comparable to that found in the human lung and heart, while the adult brain had a level several times greater than the liver.
Cho et al. (1999a) point out that the expression of these enzymes can vary greatly among individuals. The authors hypothesize that this might be due to age or, more likely in their view, regulation of the enzymes in response to the dietary intake of fatty acids.
Using cross-sectional data based on the percentage of plasma phospholipids, Welch et al. (2008, United Kingdom) estimated that non-fish-eaters (both vegetarians and meat-eaters) convert ALA to long-chain omega-3s at about a 22% higher rate than fish-eaters.
Dietary DHA Reduces ALA Conversion
In a series of three studies, researchers used a carbon tracer to track the conversion of a 700 mg dose of ALA to long-chain omega-3s in the blood of three different groups of people. The results are in the table below. Only females (all of whom were of reproductive age) showed a substantial conversion of ALA to DHA in the blood.
In addition to the baseline measurements listed in the table above, Burdge et al. (2003) included an 8-week intervention on three groups of older men: a control group (n=5), a group whose daily ALA was increased from their normal intake of 1.7 g to 10 g (n=4), and a group whose daily EPA+DHA was increased from their normal intake of 264 mg to 1.6 g (n=5). After 8 weeks, they fed each person 700 mg of ALA with a carbon tracer and found that the ALA supplemented group’s conversion of ALA to long-chain omega-3s hadn’t increased whereas the EPA+DHA supplemented group’s conversion had decreased.
Vermunt et al. (2000) fed carbon-labeled ALA to humans and found that the conversion of ALA to EPA, DPA, and DHA was much greater after 9 weeks of a diet high in oleic acid compared to after a diet high in ALA or EPA+DHA.
The two trials mentioned above by Burdge et al. (2003) and Vermunt et al. (2000) suggest that there’s a down-regulation of ALA conversion to long-chain omega-3s in humans who have a regular supply of ALA or EPA and DHA. The simplest explanation for this down-regulation is that their tissues had sufficient long-chain omega-3 levels.
Further evidence for enzymatic regulation due to dietary intake is a study by Metherel et al. (2019) who conducted a randomized controlled trial using carbon-labeled DHA. While plasma levels of EPA increased, it wasn’t due to DHA being converted to EPA, suggesting that the dietary supply of DHA resulted in the down-regulation of the conversion of EPA to DHA.
Burdge and Wootton’s data (2002) showed an uneven distribution of omega-3 fatty acids among the different components of plasma lipids (cholesterol esters, phosphatidylcholine, triglycerides, and non-esterified fatty acids). They surmised that plasma cholesterol esters act as a long-term source of ALA within circulation that may provide tissues containing active desaturation and elongation pathways (brain, heart, and skeletal muscles) a steady source of ALA for conversion to EPA, DPA, and DHA while tissues with low expressions of these enzymes, such as the kidney and pancreas, may be dependent upon the supply of pre-formed EPA, DPA, and DHA.
Lower Omega-6 Intake is Associated with Higher Serum EPA and DHA
The traditional way vegetarians have been encouraged to raise blood EPA and DHA levels is by increasing ALA and decreasing the omega-6 fatty acid, linoleic acid (LA). This is because the enzymes that convert ALA into EPA and DHA also convert the omega-6 fatty acids and there is competition for these enzymes. Some evidence for this theory is from a clinical trial by Liou et al. (2007, Canada) who found increasing LA intake resulted in a lower percentage of EPA in plasma phospholipids.
Most vegetable oils are high in omega-6s and vegetarians tend to get plenty in their diets. Sanders and Younger (1981, United Kingdom) found a dietary ratio of omega-6s to omega-3s of 16 for vegans and 6 for meat-eaters. Sanders and Roshanai (1992, United Kingdom) found a dietary ratio of 15.8 for vegan men, 10.2 for meat-eating men, 18.3 for vegan women, and 8.2 for meat-eating women.
For a discussion of the Salvador et al. (2019, Spain) paper, see Appendix D: Salvador, 2019.
At this time, it’s not clear if changing the dietary ratio of LA to ALA impacts the blood levels or, more importantly, tissue levels of EPA or DHA.
Low Omega-6 to Omega-3 Ratio Foods
Traditionally, mainstream vegan health professionals have encouraged vegans to lower their dietary omega-6 to omega-3 ratios. In part, this was due to concerns about potential inflammation caused by high LA intakes which have not borne out.
My view is that there isn’t enough evidence to avoid foods high in ALA due to concerns about the amount of LA those foods might contain. We’ve included the chart below of foods high in ALA and their ratio of LA to ALA to be thorough or in case future research suggests it’s important to choose foods with a low ratio.
| Foods with a Low LA to ALA Ratio | ||
|---|---|---|
| Food | n-6:n-3 ratio | ALA |
| Flaxseeds | 1:4 | 1.6 g / tablespoon |
| Flaxseed oil | 1:4 | 2.5 g / teaspoon |
| Chia seeds | 1:3 | 5 g / oz |
| Camelina oil | 1:1.5 (Budin, 1995) | |
| Canola oil | 2:1 | 1.3 g / tablespoon |
| English walnutsa | 4:1 – 5:1 | 2.6 g / oz (14 halves) |
| Hemp oil, refined | 1:0.3 (Mikulcová, 2017) | |
| Hemp oil, unrefined | 1:0.4 (Mikulcová, 2017) | |
| Walnut oil | 5:1 | 1.4 g / tablespoon |
| Soybean oil | 7.5:1 | .9 g / tablespoon |
| Black walnuts | 10:1 | .9 g / oz |
| aEnglish are the typical walnuts found in most grocery stores. | ||
DHA Supplementation in Vegetarians
Studies consistently show that supplementing vegetarians and vegans with DHA from algal sources increases their blood percentage of DHA (Sanders, 2009; Geppert, 2006; Wu, 2006; Conquer, 1996; Conquer, 1997). Studies also show that supplementing with both EPA and DHA increases their percentages (Sarter, 2015; Mezzano, 2000).
Fish contains about twice as much DHA as EPA (Kris-Etherton, 2009), so it’s not unusual for fish-eaters to eat more DHA than EPA. Conquer and Holub (1996, Canada) showed an 11–12% increase in EPA after 6 weeks of 1,620 mg of DHA in vegetarians.
Upon DHA supplementation, EPA levels also increase by a small percentage. Using a carbon tracer, Brossard et al. (1996, France) found a 1.4% conversion of DHA to EPA in three people given one dose of 123 mg of DHA over the course of 20 hours. In contrast, Metherel et al. (2019, Canada) conducted a randomized controlled trial using DHA containing labeled carbon and didn’t find any to be converted to EPA. They conclude that “the increase in plasma EPA following DHA supplementation in humans does not occur via retroconversion, but instead from a slowed metabolism and/or accumulation of plasma EPA.”
Omega-3 Recommendations for Vegans
To sum up the rationale behind our recommendations, it appears that if a vegan is meeting the Dietary Reference Intake for ALA, their EPA status should be adequate. To be cautious we recommend either increasing ALA intake or adding a DHA supplement.
There are many vegan DHA and EPA supplements available via the Internet. We aren’t able to assess whether any given company is better than another.
Vegetarian Pregnancy, Nursing, and Infants
This section provides our omega-3 recommendations for vegetarians and vegans during pregnancy, nursing, and infancy, followed by a discussion of the research.
Summary and Recommendations
The United States and Canadian Dietary Reference Intakes don’t specifically recommend a DHA supplement during pregnancy or lactation. Their recommendations are for an extra 300 mg of ALA during pregnancy (for a total of 1.4 g/day) and an extra 200 mg of ALA during nursing (for a total of 1.3 g/day). Their recommendations for non-breastfeeding infants is for an infant formula with 500 mg of total omega-3s (ALA, EPA, and DHA) per day (National Institutes of Health, 2022).
However, a number of organizations have superseded the DRIs by specifically recommending DHA for the general public during pregnancy and/or nursing, including the International Society for the Study of Fatty Acids and Lipids (ISSFAL) and the American Academy of Pediatrics (AAP).
The most recent Cochrane analysis of randomized controlled trials found a benefit of DHA supplementation in preventing early preterm births among apparently healthy, pregnant women (Middleton, 2018). At VeganHealth, the purpose of our recommendations are for preventing nutrient deficiencies more than for treating disease. The finding that DHA reduces the risk of a small percentage of preterm births is possibly due to a therapeutic effect of DHA rather than from correcting a nutrient deficiency. If this is the case, then meeting the DRIs for ALA might prevent an omega-3 deficiency during pregnancy and nursing, but without more research, erring on the side of caution is prudent.
Our recommendations for pregnancy, nursing, and infants are:
- Maternal diet during pregnancy
- Meet the DRIs for ALA of 1.4 g per day (see Daily Needs for sources).
- Supplement with 300-600 mg of DHA per day (Carlson, 2013; Harris, 2015).
- Maternal diet during nursing
- Meet the DRIs for ALA of 1.3 g per day (see Daily Needs for sources).
- Evidence isn’t convincing for DHA supplementation, but some organizations recommend at least 200 mg per day (Koletzko, 2007; Meek and Noble, 2022).
- Formula-fed infants
- Use a formula with 500 mg of total omega-3s (ALA, EPA, and DHA) per day; EPA and DHA are not specifically required if there’s 500 mg of ALA.
- Children eating only solid food
- Meet the DRIs for ALA (see Daily Needs for amounts and sources).
Research on Pregnant and Nursing Vegans
Ureta-Velasco et al. (2023, Spain) compared the breast milk of 20 vegetarians (including 11 vegans) to that of omnivores. The authors describe their study as one of the most complete investigations available due to having a 5-day dietary record, multiple milk samples from each participant, and measuring plasma levels of nutrients. However, the study was conducted to determine the quality of breast milk of vegetarians for donation purposes and the milk was processed and stored as donated breast milk would be; the authors said this made the results “not superimposable” to those of breast milk fed directly to infants.
Intakes of ALA were 1.58 g/day for meat-eaters and 2.24 g/day for vegetarian mothers. Among the vegetarian group, 30% had used omega-3 supplements during pregnancy and 35% were using during lactation. Total EPA plus DHA intake, including from supplements, was .53 g/day for omnivores and .14 g/day for vegetarians. Shown in the chart below, DHA in the vegetarians’ erythrocytes, plasma, and breast milk was significantly lower.
The authors write:
However, we do not consider this to be a reason for the exclusion of vegetarian/vegan women as milk donors. Firstly, the intakes and levels of DHA in human milk vary widely across countries. Indeed, values equal or even lower than those of our Veg group have been reported both in developed and developing countries. In fact, several studies on [donor human milk] in North America found DHA values that were similar to or even lower than those of our Veg group. Secondly, since DHA supplementation to lactating women has consistently been shown to increase DHA concentrations in breast milk, it seems an appropriate strategy to recommend DHA supplementation from algal oil to Veg women who want to become milk donors.
Specker et al. (1987, USA) compared the fat content of the breast milk of 7 nonvegetarian women to 12 women on a macrobiotic diet (mostly vegan with some occasional animal foods including seafood). The percentage of fat as DHA in the women’s breast milk was similar (0.27% for the macrobiotic women and 0.29% for the nonvegetarians).
Reddy and Sanders (1994, United Kingdom) measured the DHA levels in the umbilical cords of 32 infants born to Hindu vegetarian mothers living in North London and compared them to omnivores. The vegetarians had a lower percentage of DHA cord plasma phospholipids (4.0% vs. 5.8%) and cord artery phospholipids (4.1% vs. 5.8%). The Hindu vegetarian infants were born with lower body weight, length, and head circumference but there was no relationship between those parameters and the percentages of DHA in their cord plasma or cord artery phospholipids.
Reddy and Sanders (1994, United Kingdom) also measured the percentage of fat as DHA in the breast milk of 19 vegans (0.14%), 5 vegetarians (0.30%), and 21 omnivores (0.37%).
Perrin et al. (2018, USA) found that the ALA in breast milk was significantly higher in vegans (2.1%) than vegetarians (1.4%) and meat eaters (1.2%). They found no statistically significant differences between the DHA levels in 26 vegans (0.14%), 22 vegetarians (0.17%), and 26 meat-eaters (0.18%). DHA was undetectable in 14.9% of samples; there was no difference in the prevalence of undetectable samples between the diet groups. The authors note:
Two independent reviews of worldwide DHA breast milk concentrations reported averages of 0.32–0.37%, with concentrations in US populations often reported at 0.20% or lower, which is similar to our findings. While use of a DHA supplement is well supported in the literature as a way to increase breast milk DHA, less than one-third of vegans in our study reported using DHA supplements. Similarly, fish consumption was low, with only 3/26 (11.5%) of omnivores and 0/22 (0.0%) vegetarians reporting more than weekly consumption.
Because term infants as young as 3 weeks can convert ALA to DHA (Sauerwald, 1996), it’s not clear if lower percentages of DHA in vegan breast milk are of physiological significance.
Research on Omega-3 Supplementation in Pregnancy, Lactation, and Infancy
Dozens of clinical trials have measured the impact of DHA supplementation on infant development. Some studies provide DHA directly to the infants while others provide the DHA to their pregnant or nursing mothers.
There are two large systematic reviews on the topic:
- Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review (Newberry et al., 2016), prepared for the Agency for Healthcare Research and Quality (AHRQ) of the U.S. Department of Health and Human Services. This over 800-page report provides an analysis of 95 randomized controlled trials and 48 observational studies.
- Omega-3 Fatty Acid Addition during Pregnancy (Middleton, et al., 2018), a Cochrane Database Systematic Review. This over 400-page report provides an analysis of 70 randomized controlled trials.
They come to somewhat different conclusions, especially regarding the risk for preterm births.
The AHRQ report (Newberry, 2016) found that evidence was sufficient to draw a conclusion of low or moderate strength for the following outcomes:
- Supplementation with DHA increased the length of gestation but didn’t decrease the risk for preterm birth.
- Prenatal DHA supplementation increased the birth weight among healthy term infants but didn’t decrease the risk for low birth weight.
- Prenatal DHA supplementation didn’t increase the risk of gestational hypertension or preeclampsia among high-risk women.
- DHA supplementation did not impact post-delivery infant growth patterns (weight, length, or head circumference).
- Prenatal supplementation with DHA had no effect on visual acuity.
- For term infants, data conflicted on the effectiveness of supplementing infant formula with omega-3 fatty acids depending on when and how the visual acuity was assessed and the type of omega-3 provided.
- Omega-3 supplementation had no consistent effect on neurological development.
- Supplementing or fortifying infant formula for term infants with omega-3s had no effect on cognitive development.
- Omega-3 supplementation had no effect on the development of autism spectrum disorder, attention deficit hyperactivity disorder, atopic dermatitis, allergies, or respiratory disorders.
- Maternal DHA or EPA supplementation had no effect on the risk of peripartum depression.
The authors couldn’t rule out the possibility that supplementation can help those at higher risk of poor outcomes or people with low DHA levels at baseline:
Few studies stratified outcomes according to risk groups, so it was usually not possible to assess whether the effectiveness of omega-3 interventions depended on level of risk. In addition, no RCTs stratified outcomes by baseline n-3 FA status, so it is not possible to assess whether adequacy of n-3 FA status might account for differences in outcomes across (or lack of outcomes within) studies.
The Cochrane Database review (Middleton, 2018) found few statistically significant impacts of omega-3 supplementation except for a reduced risk of preterm births and low birth weight. Non-statistically significant trends were for supplementation to reduce the risk of perinatal infant death and neonatal care admission, and an increased risk of large-for-gestational-age babies. We’ll discuss the preterm births < 34 weeks in more detail as it was the most promising finding.
Long-chain Omega-3s and Preterm Births
The Cochrane report found a lower risk of preterm birth at < 37 weeks for women taking omega-3 (11.9% versus 13.4%; RR 0.89, 95% CI 0.81-0.97; 26 RCTs, 10,304 participants; Middleton, 2018). The report included at least one study that used ALA rather than EPA or DHA (Mardones, 2008); I recalculated the numbers after removing that study and still found a statistically significant impact (RR 0.89, 95% CI 0.80-0.99; Number Needed to Treat (NNT) 66, 95% CI 34-688; determined using an online calculator).
We’ll forcus on Cochrane’s finding for preterm birth < 34 weeks because it was stronger (2.7% vs. 4.6%; RR 0.58, 95% CI 0.44-0.77; 9 RCTs, 5,204 participants) and the consequences are potentially more harmful.
There are three reasons why thee Cochrane report found a stronger benefit for DHA on preterm births than did the AHRQ report:
- The data on omega-3s reducing the risk of preterm births < 34 weeks is stronger than data reducing the risk of preterm births < 37 weeks, but the AHRQ report only analyzed the latter.
- The Cochrane analysis included one study, Mardones et al. (2008) that used ALA, rather than DHA, which had a strong finding in reducing the risk of early preterm births (RR 0.19, 95% CI 0.04-0.88) whereas the AHRQ analysis didn’t include studies using ALA.
- The AHRQ report separated their analysis into studies of DHA only (OR 0.87, 95% CI 0.66-1.15) and studies of DHA plus EPA (OR 0.86, 95% CI 0.65-1.15); dividing the findings into two groups likely weakened the statistical significance.
The Cochrane report also included three studies of women with diabetes (Horvaticek, 2017; Min, 2014; and Min, 2016), a study of women with a history of preterm birth (Bulstra-Ramakers, 1994), and women at high risk for pregnancy complications (Olsen, 2000). I compiled the studies used by the Cochrane report in the spreadsheet, Preterm Delivery. After removing these five studies and the study using ALA, a benefit for DHA in preventing preterm delivery < 34 weeks remained (RR 0.49, 95% CI 0.24-0.71); the number needed to treat (NNT) is 60 (95% CI 38-150). I also calculated the statistics for the two studies that only included apparently healthy participants and for which gestational length was one of the primary outcomes: RR 0.24, 95% CI 0.09-0.67; NNT 25, 95% CI 15-72 (Carlson, 2013; Harris, 2015).
Based on these studies, it appears that DHA supplementation can help prevent preterm births before 34 weeks. Could ALA also prevent them?
ALA Intake and Preterm Births
Mardones et al. (2008, Chile) provided low-income, pregnant women with a micronutrient-fortified milk powder. The women in the treatment group also were given ALA and LA. Using an intention-to-treat analysis (the analysis used by the Cochrane report), the treatment group had a lower risk of delivery before 34 weeks (RR 0.19, 95% CI 0.04-0.88; NNT 59, 95% CI 32-334). However, if you use the on-treatment analysis (women who followed the protocol more closely), the finding for ALA is weaker due to fewer preterm births in the placebo group (RR 0.23, 95% CI 0.05-1.07; NNT 54, 95% CI 28-1,117). It’s also possible that vitamin B6 could be a factor as the placebo group averaged below the DRI (1.64 ± 0.66 mg) while the treatment had a 30% higher intake (2.14 ± 1.21) which is above the DRI of 1.9 mg for vitamin B6 during pregnancy.
Knudsen et al. (Denmark, 2006) conducted a randomized, controlled study of fish oil or flax oil supplementation among over 3,000 pregnant women with low fish intakes. The treatment groups consisted of daily supplements of 100 mg EPA plus DHA, 300 mg EPA plus DHA, 700 mg EPA plus DHA, 1,400 mg EPA plus DHA, 2,800 mg EPA plus DHA, and 2,600 mg ALA. Supplementation didn’t start until an average of 22 weeks of pregnancy and continued until delivery. No differences in the length of gestation were detected between the fish oil or flax oil groups compared with a control group that received no intervention. When the results were limited to women with a usual dietary intake of < 150 mg per day of EPA plus DHA, there was no indication that omega-3 supplementation increased the length of gestation based either on an intention-to-treat or an on-treatment analysis. The length of gestation in a control group of women eating < 150 mg of EPA plus DHA per day was 40 weeks, suggesting that this population was not at risk for preterm births despite their low long-chain omega-3 intakes.
de Groot et al. (2004) conducted a double-blind, randomized, controlled intervention trial from week 14 of pregnancy until delivery. The subjects’ baseline daily ALA intake (at 14 weeks of pregnancy) was 1.3 g. The treatment group received 2.8 g of ALA plus 9.0 g of LA daily compared to only 10.9 g/day of LA for the control group. The control group’s ALA intake was measured again at week 36 and was 1.0 g/day. The treatment group had an average gestation length of 4.5 days longer than the controls which wasn’t statistically significant (P = .091). The incidence of preterm births wasn’t measured.
More research is needed to determine whether meeting the DRIs for ALA or increasing ALA intake beyond the DRIs, is as effective as DHA in preventing preterm births.
Timing of Intervention on Early (< 34 weeks) Preterm Births
Some of the studies on DHA supplementation and preterm births started the intervention rather late in pregnancy. Our spreadsheet, Preterm Delivery, lists the timing of the interventions with no clear pattern between the timing and a beneficial finding.
Of the three studies using ALA, one started the intervention at 11 weeks and found a statistically significant result (Mardones, 2008), one started at 14 weeks and found a non-significant trend (de Groot, 2004), and one started at 22 weeks with no positive impact (Knudsen, 2006). This might be coincidental.
Official Recommendations
As of November 2022, the United States and Canada Dietary Reference Intakes don’t specifically recommend a DHA supplement during pregnancy or lactation.
There are other organizations that recommend DHA during pregnancy and nursing. Here I review the evidence used by the International Society for the Study of Fatty Acids and Lipids (ISSFAL) in some detail and also include the recommendations from the American Academy of Pediatrics.
ISSFAL Omega-3 Recommendations During Pregnancy and Nursing
In 2007, many organizations, including the International Society for the Study of Fatty Acids and Lipids (ISSFAL) published a Consensus Statement that recommends DHA intake of an average of at least 200 mg per day during pregnancy and nursing. They used two lines of reasoning.
The first line of reasoning is that the conversion of ALA to DHA is limited:
The fractional conversion of a-linolenic acid to n-3 LC-PUFA may be greater in women than in men, which may contribute to meeting the demands of the fetus and the breastfed neonate for DHA, but most evidence indicates that the overall contribution of a-linolenic acid to DHA is limited; therefore, adequate intakes of preformed n-3 LC-PUFA, and in particular DHA, appear important for maintaining optimal tissue function (5-7).
For this claim, citation 5 was a review paper by JT Brenna (2022) who is actually one of the authors of the Consensus Statement. However, I didn’t find Brenna’s review paper to provide evidence in favor of the statement’s claim. On the contrary, Brenna says:
Failure to elevate 22:6n-3 [DHA] blood-compartment concentrations by 18:3n-3 [ALA] supplementation does not necessarily mean that 22:6n-3 concentrations do not increase in tissues. Conversion and conservation of 18:3n-3 may be efficient in developing neural tissue and in very active tissues such as retina which actively recycles 22:6n-3. Reliable measurements of these processes, however, are not possible without access to tissue samples and therefore are not possible for most tissues in humans. Animal studies of conversion efficiency are of limited value because of physiological differences, as cited below.
Citation 6 was a paper by GC Burdge (2005) which says:
Overall, [ALA] appears to be a limited source of longer chain n-3 PUFA in humans. Thus, adequate intakes of preformed long chain n-3 PUFA, in particular DHA, may be important for maintaining optimal tissue function.
However, as reviewed in this article’s appendix, Evolutionary Arguments for a Dietary Requirement for DHA, Burdge subsequently published a paper in 2022 in which he argues that ALA is an adequate source for meeting the omega-3 needs of infants (Burdge, 2022).
Citation 7, a review by SM Innis (2005), provides an analysis of DHA amounts in the brain and argues that preterm infants cannot produce enough DHA to supply the brain with this amount. But the review seems to suggest that, at most, we don’t know whether term infants can benefit from DHA supplementation.
The Consensus Statement’s second line of reasoning is based on the clinical trials of DHA supplementation during pregnancy and nursing which the authors believed showed beneficial effects on infant development. They cite eight clinical trials, all of which are included in the 2016 report from the Agency for Healthcare Research and Quality reviewed above (Newberry, 2016). I discuss this and subsequent clinical trials in detail above.
American Academy of Pediatrics DHA Recommendations during Nursing
In their Technical Report: Breastfeeding and the Use of Human Milk, the American Academy of Pediatrics (AAP) says, “The mother’s diet should include an average daily intake of 200 to 300 mg of the omega-3 long-chain polyunsaturated fatty acids for adequate preformed docosahexaenoic acid DHA in the mother’s milk and to improve the infant’s fatty acid status (Meek and Noble, 2022).”
The AAP doesn’t provide a rationale for their recommendation in this Technical Report, but the rationale they provide in their 2019 edition of Pediatric Nutrition doesn’t include anything important that wasn’t covered here. The AAP doesn’t provide recommendations for pregnancy.
Omega-3s and Chronic Disease
Most of the concern with regard to low plasma levels of EPA and DHA among vegetarians is due to studies that have found an association between low EPA and DHA blood levels and an increased risk of chronic diseases such as cardiovascular, cognitive decline, and depression. These associations have generally been consistent but weak. There have also been some associations between omega-3 blood levels and an increase in some chronic diseases. In this section we review the evidence.
Omega-3s and Cardiovascular Disease
Research on omega-3s and cardiovascular disease has examined the associations with fish consumption, blood levels of omega-3s, and omega-3 supplementation.
Fish Consumption and Cardiovascular Disease
As of February 2021, the American Heart Association was still basing its omega-3 fatty acid recommendations on its 2002 position paper, Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease (Kris-Etherton, 2002) which recommends that adults “Eat a variety of (preferably oily) fish at least twice a week. Include oils and foods rich in alpha-linolenic acid (flaxseed, canola, and soybean oils; flaxseed and walnuts).”
Some recent reports include:
- A 2020 meta-analysis of six cohort studies found no correlation between eating fish and a reduced risk of cardiovascular disease or mortality (Zhong, 2020).
- A 2020 Cochrane review determined that there wasn’t enough evidence to assess the impact of eating fish on cardiovascular health (Abdelhamid, 2020).
- A 2016 meta-analysis of 12 prospective studies found a reduced risk of mortality with increasing fish intake (Zhao, 2016).
Omega-3 Supplementation and Cardiovascular Disease
In what they called “the most extensive systematic assessment of effects of omega-3 fats on cardiovascular health to date,” a 2020 Cochrane Review analyzed 86 randomized controlled trials of 12 to 88 months duration using omega-3 capsules, omega-3-enriched food, or dietary advice to eat more omega-3s (Abdelhamid, 2020). The review found little to no effect of increasing omega-3s on all-cause or cardiovascular mortality, cardiovascular events, stroke, or arrhythmias. Increased omega-3 intake showed a trend with reduced coronary heart disease mortality (RR 0.90, CI 0.81-1.00) and there was a reduced rate of coronary heart disease events (RR 0.91, CI 0.85-0.97). Increasing long-chain omega-3s reduced triglycerides by ~15% in a dose‐dependent way. Overall, the authors stated that 334 people would need to increase their long-chain omega-3 intake to prevent one person from having a coronary heart disease event and they believed this wasn’t enough of an impact to recommend supplementation.
In contrast, a 2019 meta-analysis of omega-3 supplementation found a benefit from omega-3 supplementation in the combined results from 13 randomized controlled trials using about 800 to 1,800 mg of omega-3 fatty acids per day (Hu et al.). At baseline, the participants had a mixed risk for cardiovascular disease: 40% had diabetes and 73% were using cholesterol-lowering medication. In one set of results, that excluded the REDUCE-IT trial described below, they found a reduced risk of heart attack (RR 0.92, CI 0.86-0.99) and cardiovascular death (RR 0.93, CI 0.88-0.99). The omega-3 supplementation in this set of results is arguably higher than the AHA recommendations of at least 2 servings of fish per week, but not implausible. For the omega-3 content of fish, see Omega-3 Fatty Acids: Fact Sheet for Health Professionals.
The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) was excluded from Hu et al.’s results above because it used a much higher dose of omega-3s: 4,000 mg/day of a purified form of EPA. It showed markedly better success for heart attack (RR 0.69, CI 0.58-0.81) and cardiovascular death (RR 0.80, CI 0.66-0.98). Participants also had a lower risk of stroke (RR .72, CI 0.55-0.93), but their death from all causes wasn’t significantly lower (RR .87, CI 0.74-1.02) than the placebo (Bhatt, 2019). The extremely high amount of EPA used in REDUCE-IT is a pharmacological dose and not relevant to dietary omega-3 intake.
Stroke and Omega-6 to Omega-3 Ratio
Cupino et al. (2022) analyzed the ratio of omega-6 to omega-3 intakes and risk of stroke among the Adventist Health Study-2 cohort. They found a statistically significant association between omega-6 to omega-3 ratio and an increased risk of any type of fatal stroke (HR 1.40, 95% CI: 1.16-1.69) for the 90th percentile ratio (12:1) vs. 10th percentile ratios (6:1).
Omega-3s and Cognition
A 2012 cross-sectional report from the Framingham Study examined 1,575 people (54% women) with an average age of 67 (SD 9) years with respect to omega-3 blood levels and numerous cognitive-related parameters (Tan, 2012). They compared the EPA+DHA red blood cell membrane fatty acids in the lowest quartile (≤4.4%) to those in the upper three quartiles (75th percentile was 6.5%). They found that those in the lowest quartile had a significantly lower cerebral brain volume (equivalent to approximately two years of brain aging), but a similar white matter hyperintensity volume, temporal horn volume, and rate of silent stroke. Low blood EPA+DHA was associated with a poorer score on some tests of cognition.
As part of the Women’s Health Initiative Study of Cognitive Aging, Ammann et al. (2013, USA) conducted a cross-sectional analysis of 2,302 women 65 years and older and found no difference in cognition between those in the upper one-third compared to those in the lowest one-third of EPA+DHA percentage of fatty acids in red blood cells. However, the lowest one-third had an average EPA-plus-DHA of 3.8% which is quite a bit higher than vegans tend to have, so this finding doesn’t necessarily reassure us about the omega-3 status of vegans. A 2017 study by Ammann et al. (described below), followed a much larger group of participants over time and provides more insight into whether higher EPA and DHA percentages are important in preventing cognitive impairment and dementia, especially in older women.
Zhang et al. (2016) conducted a meta-analysis of 21 case-control and prospective studies and found that increases of 1-serving/wk increments of fish were associated with a reduced risk of dementia (RR 0.95, CI 0.90-0.99) and Alzheimer’s disease (RR 0.93, CI: 0.90-0.95). DHA intake was also inversely associated with risks of dementia (RR 0.86, CI 0.76-0.96) and Alzheimer’s disease (RR 0.63, CI 0.51-0.76). However, blood levels of omega-3 fatty acids were not associated with a reduced risk of these or other cognitive diseases. In a letter to the editor, Koch and Jensen point out that in the six studies looking at the association between fish intake and dementia and Alzheimer’s disease, one study was a 2-year follow-up of another study with a longer follow-up. Koch and Jensen argue that “Appropriate exclusion of the report from Kalmijn et al. would render the meta-analysis of fish intake in relation to dementia risk insignificant (RR: 0.96; 95% CI: 0.91, 1.01; no heterogeneity) and change the RR estimate for AD risk to 0.87 (95% CI: 0.77, 0.98) in a random-effects meta-analysis with significant between-study heterogeneity still present.” Zhang and Jiao responded that it was appropriate to include both reports. It’s perplexing that omega-3 intakes but not blood levels would be associated with a reduced risk of dementia if there is a true effect, though it might suggest that blood levels of EPA and DHA aren’t an accurate representation of omega-3 status.
Amman et al. (2017, USA) conducted the largest prospective study to assess the risk of dementia with omega-3 fatty acid status. The study was part of the Women’s Health Initiative Memory Study testing the impact of the hormones estrogen and progestin on the memory of women ≥65 years old. Although the hormone part of the study was concluded early, the researchers continued to follow 6,706 women for an average of 9.8 years to see if baseline EPA and DHA levels were associated with a diagnosis of probable dementia (PD) or mild cognitive impairment (MCI). The study compared the risk of PD and MCI among those with EPA/DHA within one standard deviation above the mean (5.3-6.8% EPA+DHA) to those within one standard deviation below the mean (3.8-5.3% EPA+DHA). In one of their models, the researchers found a statistically significant reduction in PD (HR 0.91, CI .83-.99), but most models found no significant association including one that adjusted for the APOE genotype associated with Alzheimer’s Disease (HR 0.92, CI 0.83-1.01). The researchers calculated that the increased risk of PD represented a 2% reduced risk (12% vs. 14%) of PD incidence over a 15-year period. There were no significant associations between EPA+DHA and MCI. Examining EPA and DHA separately produced no significant findings.
In summary, studies of omega-3 fatty acids conducted on populations of omnivores consistently find some significant associations with better cognition, though they tend to be weak. That dietary intakes are more strongly associated with better cognition than blood levels raises a question about whether omega-3s are responsible for the beneficial association rather than other variables paired with omega-3 intake.
Omega-3s and Depression
Our interest in omega-3s and depression is mostly related to whether vegetarians are at an increased risk of depression due to lower EPA or DHA levels.
Risk of Depression
Deane et al. (2019) conducted a meta-analysis and systematic review of 32 randomized controlled trials and found no effect of increasing EPA and DHA on the risk of depressive symptoms (RR 1.01, CI 0.92-1.10). Studies had a median duration of 12 months with a median dose of 0.95 grams per day (ranging from 0.4 to 3.4 grams per day). One study addressed omega-3s and anxiety and found little to no effect. The researchers recommend against taking omega-3 supplements for reducing the risk of depression or anxiety.
Treatment for Depression
Whether EPA or DHA can be used to treat people with depression is only loosely related to the omega-3 status of vegetarians, but it’s where most of the research has focused and so I review it here.
Early research on treating depressive symptoms with supplementation of EPA and DHA was mixed. In a 2006 review, Sontrop and Campbell found that supplementation improved depression but it wasn’t clear whether it was effective for depressed patients in general or only those with abnormally low concentrations of EPA and DHA. In another 2006 review, Appleton et al. found “little support” based on the small number of trials with significant variation. In a 2007 meta-analysis Lin and Sue found a positive effect of supplementation but with significant publication bias. In a 2009 meta-analysis, Martins found evidence that EPA is more effective than DHA.
Grosso et al. (2014) conducted a meta-analysis of 11 trials of patients with a DSM-defined diagnosis of a major depressive disorder (MDD) and 8 trials of patients with depressive symptomatology but no diagnosis. They found supplementation to have a beneficial effect for the patients diagnosed with MDD and also for those with bipolar disorder. They considered EPA to be more effective, with many trials using pharmacological doses. Hallahan, et al. (2016) found similar results in their meta-analysis.
In their meta-analysis, Luo et al. (2020) found a benefit from high-dose (≥2 g/day) but not low-dose (<2 g/day), EPA/DHA supplementation in the early therapy period for MDD.
Omega-3s and Increased Risk of Disease
Some studies have associated higher omega-3 intakes with an increased risk of disease.
Prostate Cancer
A meta-analysis of randomized controlled trials (Hanson, 2020) measured the impact on the risk of a prostate cancer diagnosis of a higher long-chain omega-3 intake (7 trials, RR 1.10, 95% CI 0.97-1.24, NNTH 334) and of a higher ALA intake (2 trials, RR1.30, 95% CI 0.72–2.32, NNTH 334). Neither finding was statistically significant and had a high number needed to harm (NNTH). There was no impact on overal cancer diagnosis or death. The authors provided a chart showing that the benefits of higher omega-3 intake on cardiovascular events outweighed the potential harm.
A meta-analysis (Carleton, 2013) of case-control and prospective studies found no association between ALA intake and prostate cancer (RR 1.08, 95% CI 0.90-1.29). Removing one study during sensitivity analysis resulted in a weak, non-significant protective effect of ALA (RR 0.91, 95% CI 0.83-0.99, p=0.02).
A systematic review and meta-analysis (Simon, 2009) of ALA blood or tissue concentrations and prostate cancer found no association after adjustment for publication bias (RR: 0.96, 95% CI 0.79-1.17).
A 2010 meta-analysis found that subjects who consumed more than 1.5 g/day of ALA had a significantly decreased risk of prostate cancer (0.95, 0.91-0.99) compared to those who ate less (Carayol, 2010).
A 2018 paper from Harvard School of Public Health suggested that past associations between ALA and prostate cancer might have been due to trans-ALA which has been largely removed from the food supply (Wu, 2018).
A case-control study from the SELECT trial (Brasky, 2013) found an association between higher DHA phospholipid levels and prostate cancer. This finding is superceded by the findings of Hanson et al. (2020) described above, but a discussion of this finding from 2013 can be found on the JackNorrisRD.com blog post, DHA Supplements and Prostate Cancer.
Eyesight
A 2001 analysis from the Nurses Health Study found an almost statistically significant increase in age-related macular degeneration for those with the highest ALA intake (Cho, 2001, USA).
In contrast, a 2013 study found that higher ALA levels in the blood were associated with a lower risk of late age-related macular degeneration (Merle, 2013, France). And a 2017 follow-up from the Nurses Health Study found that a high intake of ALA was associated with an increased risk of intermediate age-related macular degeneration before 2002, but not afterward when lower levels of trans fats were found in participants’ blood (Wu, 2017, USA).
A 2005 analysis from the Nurses Health Study found that both the highest intakes of ALA and LA were associated with an increase in lens opacity, which can lead to cataracts (Lu, 2005, USA). For ALA, the risk ratio was 2.2 (1.2, 4.5) for about 1.26 g compared to .86 g per day. A 2007 analysis of the same group found that the highest category of ALA intake (about 1.26 g per day) was linked to a 16% increase in eye lens nuclear density compared to the lowest category (about .84 g per day) over five years. As of 2018, no follow-up studies appear to have been conducted on ALA and cataracts (Lu, 2007, USA).
Without more definitive research we don’t believe concerns about eyesight is any reason to avoid plant-based ALA due to the small differences in ALA intake in these studies, the fact that much ALA in meat-based diets comes from animal products, that trans ALA is no longer added to the food supply, and the large number and inconsistencies of associations between different fatty acids and various conditions.
Appendix A: Biomarkers of DHA Status
The primary reason why researchers rely on plasma and/or red blood cell percentages is convenience: they’re the most accessible and straightforward measurements to obtain. Studies consistently find inverse associations, albeit weak, between blood percentages and morbidity which bolsters their use.
In their review, Biomarkers of DHA status, Kuratko and Salem (2009) argue that the percentage of fatty acids as DHA in plasma or red blood cells is a generally adequate marker of overall DHA status. However, they also highlight many of the caveats that I discuss in Conversion of ALA to EPA and DHA.
Kuratko and Salem cite one paper worth discussing here. In a gruesome experiment, Sarkadi-Nagy et al. (2004) studied baboon infants in order to measure various fatty acids in their blood and tissues. At 14 days old, the infants were given a carbon-tracer labeled dose of ALA. After another 14 days, they were killed and the researchers measured correlations between the carbon-labeled DHA in plasma and red blood cells with carbon-labeled DHA in the brain, retina, and liver. R-coefficients are listed in the table below.
Kuratko and Salem summarize Sarkadi-Nagy et al.’s results by saying, “Both erythrocyte and plasma levels were correlated with resulting DHA content in brain, retina, and liver.” But these correlations weren’t between the total percentage of fatty acids as DHA; rather they were the percentage of fatty acids as DHA that was converted from ALA during the study period. In contrast, in their Table 3, Sarkadi-Nagy et al. list the total amounts of various fatty acids in plasma, red blood cells, and tissues and while there were some similarities there were also many differences.
Sarkadi-Nagy et al. write, “It is widely recognized that tissue and plasma [fatty acid] concentrations respond to diet. Improvements in function cannot be directly inferred and must be demonstrated (43). Furthermore, the wide variation in the regulation of individual [fatty acid] concentrations from tissue to tissue makes extrapolation difficult from compartments accessible in humans, primarily plasma and RBCs.” In other words, for their view that blood levels of DHA are adequate markers of omega-3 status, Kuratko and Salem rely on a paper by Sarkadi-Nagy et al. who disagree that tissue status can be extrapolated from blood percentages.
Kuratko and Salem also cite research on rhesus monkeys and then summarize by stating, “Although a statistical correlation was not reported, it is evident that a positive relationship exists between erythrocyte DHA levels and tissue levels, including the brain cortex and retina [68,69].” Their citation 68, Pawlosky et al. (1997), didn’t measure fatty acid levels in erythrocytes. Their citation 69, Pawlosky et al. (2001(a)), used a carbon tracer to measure the conversion of ALA to EPA and DHA in human blood but not tissues. Kuratko and Salem’s paper also includes a citation 58, Pawlosky et al. (2001(b)), which refers to the research using rhesus monkeys, but the paper wasn’t readily available and the abstract doesn’t indicate that the study would support their claim.
At the time of their writing, Kuratko and Salem were employees of Martek Biosciences which could arguably benefit from a convenient biomarker of DHA status that would err on the side of overestimating the need for DHA supplementation.
There are likely many other studies not mentioned in this article that have drawn various comparisons between percentages of long-chain omega-3 fatty acids in blood and tissues. The correlations do exist in some populations, but they are vague and cannot be used to draw any definite conclusions about the omega-3 status of apparently healthy individuals.
Appendix B: Evolutionary Arguments for a Dietary Requirement for DHA
A nutrient is essential if it must be provided by the diet for optimal health. A nutrient is conditionally essential if it’s required in the diet only under suboptimal conditions.
There’s an evolutionary argument that DHA is an essential nutrient, which goes:
For long periods of our evolution, humans lived near large bodies of water that provided a plentiful source of dietary DHA through fish. This plentiful DHA allowed the human brain to grow in size and intelligence. As a result, humans are still dependent on a source of dietary DHA for optimal brain health.
Short-chain omega-3 and omega-6 fats compete for the same enzymes to be elongated. Due to this, there’s also an evolutionary argument that DHA is a conditionally essential nutrient:
In modern diets, people eat such large amounts of omega-6 fats that the body’s ability to produce enough DHA to compete against the omega-6s is compromised. Under the suboptimal condition of a high omega-6 intake, dietary DHA becomes essential.
My focus is primarily on the first evolutionary argument—whether humans require a dietary source of DHA due to an evolutionary dependence on eating fish. However, the research I will review sometimes conflates the two arguments and so I wanted to describe them both at the outset.
There are four life stages when DHA could be either essential or conditionally essential:
- During fetal development, the fetus is at least partially dependent on obtaining DHA from the mother.
- Infants might be dependent on a dietary source of DHA from the mother’s breast milk or formula.
- During pregnancy or lactation, without a dietary source of DHA, the mother might not be able to convert enough ALA into DHA to provide for herself and her infant’s needs.
- During adulthood, a dietary source of DHA may be required to prevent cognitive decline.
Whether infants require a dietary source of DHA for optimal brain development isn’t relevant to the argument that humans have evolved with a dependence on eating fish because infants can obtain dietary DHA from breastmilk, and so I don’t spend much time addressing this question.
I also don’t address arguments about the food sources of pre-modern humans because even if pre-modern humans ate ample amounts of fish, it wouldn’t mean that modern humans have a dietary requirement for DHA.
Instead, I review the evidence for what I consider to be the most persuasive arguments for dietary DHA being essential. Some of these arguments are ecological, such as claims that human brain size and intelligence have decreased while neurological issues and mental illness have increased due to a move away from an aquatic diet. I also address specific physiological claims about the metabolism of omega-3s based on clinical research. Much of this research used animals.
Below, I review three published papers arguing that humans evolved on an aquatic diet that included fish and, therefore, included a consistent, dietary source of DHA that increased our brain size and intelligence. They imply that we still depend on dietary DHA for optimal brain development and maintenance. I also review two papers arguing that there’s no dietary need for DHA beyond infancy.
Crawford, 2006 and Crawford et al., 2022
Michael A. Crawford is a researcher with the Institute of Brain Chemistry and Human Nutrition in the U.K. He’s published scientific papers, sometimes with co-authors, arguing that omega-3s are neglected nutrients in the modern world and that a dietary source of DHA was important for the evolution of the human brain and is still important for infant brain development.
I’ll address what I consider to be the most persuasive arguments in his articles, Docosahexaenoic acid in neural signaling systems (2006) and Neurodevelopment, nutrition and genetics (2022).
Crawford’s 2006 article appears to be a response to John Langdon’s 2006 paper arguing that there’s no evolutionary requirement for DHA beyond infancy, which I review later (below). Crawford says that “[Langdon] neglects the basic principle of Darwinian evolution. The well documented greater efficiency of preformed docosahexaenoic acid for brain incorporation during development would have conferred a distinct survival advantage over those without it.”
But despite his many arguments and citations, Crawford provides little more than conjecture that a dietary source of DHA, beyond what is in breastmilk, is required for the proper development of the human brain. Instead, he cites evidence that omega-3s, which could be in the form of ALA, are required.
For example, Crawford (2006) writes that “DHA in the diet is rapidly and selectively incorporated in brain cell membranes and is concentrated at synaptic signaling sites (Suzuki et al., 1997).” In this study by Suzuki et al., male mice were deprived of omega-3s for 11 days and then placed in a diet group for either 6 or 30 days. There were numerous groups of mice, but I’ll limit this summary to the dietary groups of no omega-3s, ALA, and DHA. The table below shows the levels of DHA among the various groups of mice at the end of each trial.
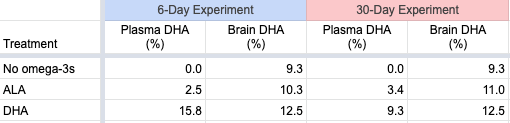
The plasma levels vary considerably, while the brain levels are similar. The physiological significance of the differences in the brain percentages of DHA between the ALA and DHA-fed groups wasn’t determined. Arguably, this study provides reassurance that, at least for mice, ALA as the only dietary source of omega-3s may be adequate. But since mice in the wild are unlikely to have evolved with a significant source of dietary DHA, these results can’t necessarily be applied to humans.
Crawford (2006) writes:
Deficiency studies in rodents (Sinclair and Crawford, 1973), primates (Fiennes et al., 1973, Neuringer et al., 1976) and visual and cognitive trials in human infants (Birch et al., 2000) have indicated that DHA is essential to brain development and function.
Sinclair and Crawford (1973) don’t isolate DHA in any way to justify this claim. Fiennes et al. (1973) studied omega-3 deficiency, not DHA deficiency. Neuringer et al. (1986) studied diets low in ALA. Birch et al. (2000) showed that infants who weren’t breastfed, but who had DHA in their infant formula, performed better on some cognitive and motor skills tests than infants without DHA in their infant formula.
On an epidemiologic level, Crawford argues that a move away from aquatic diets has caused a deficiency of important nutrients, most notably DHA and iodine, that has led to a global increase in mental disorders, and a reduction in intelligence and brain size. I’ll address these points but, because these topics are vast, I’m merely doing a cursory review.
Mental Health and Brain Disorders
Crawford (2006) writes:
The rise in mental ill health and brain disorders, to replace all other costs in the European list of burdens of ill health, raises interesting questions as to its association with the reduced availability and consumption of marine and fresh water products.
In 1972, based on the changing diet and lipid science, we predicted brain disorders would rise and this has now happened. Brain disorders now account for the highest cost to the burden of ill health in the EU (€386 billion) having overtaken heart disease, cancer and obesity by a large margin. This is hardly surprising. The brain first evolved in the sea and still uses marine fatty acids; the loss of the marine food chain is bound to have consequences.
In 2022, Crawford et al. continued to emphasize this argument saying, “One in five adults in US are reported to live with mental illness in 2019, prior to the pandemic [National Institutes of Health]; the number of 197.3 million in India suffer from mental disorders in 2017 is of the same order, double digit percentage of population [GBD India].”
Is Crawford correct that brain and mental illnesses are increasing?
According to the paper, Global, regional, and national burden of neurological disorders, 1990–2016, the absolute numbers of neurological disorders are increasing due to an aging population, but after adjusting for age and other variables, the rates are actually decreasing. For this analysis, they included a wide range of diseases, some that seem unlikely to be related to a DHA deficiency. Here’s a list of some specific diseases and the percentage of increase (+) or decrease (-) in their age-adjusted incidence between 1990 and 2016: stroke (-8%), Alzheimer’s disease and other dementias (+2%), Parkinson’s disease (+22%), idiopathic epilepsy (+6%), multiple sclerosis (+10%), and migraine (-2%).
Regarding mental illness, Crawford et al. (2022) cite the National Institutes of Health web page, Mental Illness. While the page doesn’t assess whether mental illness is increasing, they say that any mental illness (AMI) affects a large percentage of U.S. adults (21% as of 2020). AMI is defined as a mental, behavioral, or emotional disorder and can vary in impairment, ranging from none to mild, moderate, or severe. Regarding severe mental illness, the prevalence was 5.6% of all U.S. adults, with it being higher among adults aged 18-25 (9.7%) than adults aged 26-49 (6.9%) and aged 50+ (3.4%). Based on data from the National Comorbidity Survey Adolescent Supplement, 49.5% of adolescents had AMI; of those, 22.2% had severe impairment.
Regarding mental illness in India, Crawford et al. (2022) cite The burden of mental disorders across the states of India: the Global Burden of Disease Study 1990-2017 which found that the contribution of mental disorders to the total disability-adjusted life-years (DALYs) in India increased from 2.5% in 1990 to 4.7% in 2017. In 2017, depressive disorders contributed the most to the total DALYs (33.8%), followed by anxiety disorders (19.0%), idiopathic developmental intellectual disability (10.8%), schizophrenia (9.8%), bipolar disorder (6.9%), conduct disorder (5.9%), autism spectrum disorders (3.2%), eating disorders (2.2%), and attention-deficit hyperactivity disorder (0.3%). Dementia wasn’t mentioned in this report.
These rates of mental illness do seem rather high but some suggest it’s due to higher reporting. An article in The Guardian, Mental illness: is there really a global epidemic? (June 3, 2019) reports that in the previous 20 years, two variables have impacted the rates of mental illness: recognition and destigmatization. This has resulted in more people seeking help and reporting mental distress. Harvey Whiteford, professor of population mental health at the University of Queensland is quoted as saying, “All the modelling we’ve done in high-income countries where there’s survey data which has tracked over time shows that the prevalence hasn’t changed–it’s flatlined.”
The idea that brain disorders and mental illnesses are increasing due to a lack of dietary DHA is an interesting hypothesis which, at best, is difficult to assess.
Intelligence
Crawford et al. (2022) argue that worldwide average intelligence quotients (IQ) peaked in the birth year 1975 and then fell steadily through the birth year 1990. While Figure 1 in their paper shows a consistent trend, the difference is only about 2.5% of a normal IQ.
Norway is an interesting case regarding the decrease in IQ compared to per capita fish consumption. An article from the World Economic Forum, IQs are falling – and have been for years (2018), provides data from Norway where men reporting for national service between 1970 and 2009 were given IQ tests. It says “[The] analysis showed that the men born in 1962 had higher scores than those born in 1991. Those born in 1991 scored five points lower than those born in 1975, and three points lower than those born in 1962. This is the opposite of what happened during much of the 20th century when IQ scores rose by around 3% a year.”
Norway has one of the highest per-capita fish intakes of any country. The HelgiLibrary created a graph using FAOSTAT data, Fish Consumption Per Capita in Norway, showing an increase from about 41 kg in 1960 to about 53 kg in 2013. The increase in per-capita fish consumption in Norway during the time that IQ was decreasing suggests that the decrease in IQ was not due to a drop in fish consumption.
Norway was the only country for which I found data accessible enough to make this comparison. Based on this data from Norway, it seems unlikely that decreases in intelligence are due to a move away from an aquatic-based diet.
Brain Size
Crawford et al. (2022) write:
Dr Marta Lahr from the UK Cambridge University’s Leverhulme centre for Human Evolutionary Studies presented her findings to the Royal Society in 2013 that the brain size of the modern-day humans has been shrinking since the beginning of the land-based agriculture and animal husbandry 10,000 years ago.
For this statement, Crawford et al. cite a short news article, Farming to blame for our shrinking size and brains (by Deborah Braconnier, Phys.org, 2011). This article ends with:
Agriculture however does not explain the reduction in brain size. Lahr believes that this may be a result of the energy required to maintain larger brains…This reduction in brain size however does not mean that modern humans are less intelligent. Human brains have evolved to work more efficiently and utilize less energy.
A related article, Are brains shrinking to make us smarter? (by Jean-Louis Santini, Phys.org, 2011) says:
But the downsizing does not mean modern humans are dumber than their ancestors — rather, they simply developed different, more sophisticated forms of intelligence, said Brian Hare, an assistant professor of anthropology at Duke University.
A 2019 article from Discover, The Human Brain Has been Getting Smaller Since the Stone Age (by Bridget Alex), says that the most likely explanation for a reduction in human brain size is the survival of the friendliest:
The idea is, within Stone Age societies, cooperative, level-headed individuals were more likely to survive and reproduce than combative, aggressive ones. Those pro- or anti-social inclinations were influenced by genes regulating hormones, which also affected physical traits, including body and brain size. Over time, “survival of the friendliest” led to humans with slighter builds and brains on average. So although there was a reduction in skull size—and possibly intelligence—human cooperation grew, cultivating greater collective wisdom.
I don’t consider these news articles to be scientifically rigorous, but they suggest that a move away from an aquatic diet isn’t the accepted explanation for a reduction in brain size and that this reduction in brain size isn’t indicative of a health crisis.
Genetics and the n6:n3 Ratio
Crawford et al. (2022) propose a theory that traditional vegetarian societies, such as in the Pune region of India, tend to have genetic variants for fatty acid conversion that lead to low conversion of ALA to DHA when omega-6 intakes are high (such as in modern diets), regardless of how much ALA is added to the diet. If true, DHA would be conditionally essential in these contexts, but this is different from the claim that humans evolved with a dietary requirement for DHA due to a reliance on an aquatic-based diet.
Bradbury, 2011
In 2011, Dr. Joanne Bradbury from the School of Health and Human Sciences of Southern Cross University in Australia, published the paper, Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain.
Bradbury argues that modern humans have adapted to a dietary intake of preformed DHA. Bradbury’s arguments are based on the idea that endogenous synthesis of DHA from ALA in humans is “much lower and more limited than previously assumed.” Bradbury attributes the supposedly limited conversion of ALA to DHA to an increase in omega-6 intake. She also cites research with infants suggesting that dietary DHA is critical for their development, which, as I point out above, is available through breastmilk and doesn’t provide evidence for an evolutionary need for eating fish.
Langdon, 2006
In 2006, John Langdon of the Departments of Biology and Anthropology at the University of Indianapolis wrote a paper, Has an aquatic diet been necessary for hominin brain evolution and functional development?, arguing that an aquatic food supply was not critical for the evolution of the human brain nor is DHA essential for the brain development of children today.
Langdon reviews a wide range of topics. For example, he cites a survey finding that of 123 modern hunter-gatherer groups, fishing didn’t provide a measurable part of the diet in 23.
In my opinion, this excerpt sums up Langdon’s strongest argument:
An overall body deficiency [of DHA] permits DHA to be substituted by other fatty acids within the membranes, including docosapentaenoic acid (22:5n-6) [omega-6 DPA] or arachidonic acid (20:4n-6) [AA], so that body tissue composition can shift fairly quickly as diet changes (Neuringer et al. 1988; Uauy, 1990; Neuringer, 1993). This replacement may be temporary, until body levels of DHA are restored, and it implies that temporary shortages of DHA need not interfere with the rate of overall brain growth.
Let’s see what evidence Langdon cites to support this view.
I’m not sure why Langdon cites the 1993 paper by Neuringer. In that brief review, Neuringer describes a study in which infants who had been fed formula without DHA had lower levels of DHA (7.0-7.5%) in their cerebral cortex compared to those who received breastmilk (~9.75%). This was despite the fact that one group of formula-fed infants was older (16-43 weeks) than the breastfed infants (5-16 weeks) and that DHA normally accumulates in the brain as infants age. This study didn’t address whether omega-6 DPA or AA could adequately replace DHA and, therefore, doesn’t support Langdon’s argument.
The 1988 review by Neuringer et al. seems more relevant. They say:
As first observed by Mohrhauer & Holman, decreases in DHA were compensated by increases in the n-6 fatty acids 22:5n-6 and, to a lesser extent, 22:4n-6. Consequently, the total level of 22-carbon polyunsaturated fatty acids remained roughly constant, and the degree of polyunsaturation changed only slightly. This reciprocal replacement of DHA by long-chain n-6 fatty acids is a consistent finding in studies of n-3 fatty acid deficiency. It is particularly striking because, with a few exceptions, 22:5n-6 is normally present at very low levels in animal tissues. Therefore, the ratio of 22:5n-6 to 22:6n-3 in blood and tissues has been suggested as an index of n-3 fatty acid deficiency [Galli et al., 1974].
Neuringer et al. strongly imply that replacing DHA with omega-6 DPA isn’t optimal. In examining their citations, the study by Mohrhauer and Holman (1963) used rats and didn’t assess any potential problems with substituting the long-chain omega-6 fatty acids for DHA. Similarly, the study by Galli et al. used rats and didn’t assess the impacts of substituting long-chain omega-6 fatty acids for DHA.
Finally, the 1990 review by Uauy cited by Langdon doesn’t provide evidence to support Langdon’s statement. Uauy actually argues that infants require a dietary source of DHA.
While I’m sympathetic to much of what Langdon writes and would prefer that it be true, the most critical part of his paper doesn’t convince me.
Burdge, 2022
Graham Burdge is a lipid researcher from the University of Southampton in the U.K and was the lead researcher on a series of omega-3 conversion studies (Burdge et al., 2002; Burdge and Wootton, 2002; Burdge et al., 2003).
In 2022, Burdge published a paper, α-linolenic acid interconversion is sufficient as a source of longer chain ω-3 polyunsaturated fatty acids in humans: An opinion. In this thorough review article, Burdge argues that a dietary intake of ALA is sufficient to meet the body’s needs for EPA and DHA.
Rather than being concerned that vegetarians might need to supplement with DHA, Burdge argues that the apparently healthy status of long-term vegetarians and vegans suggests that there is no dietary need for EPA and DHA.
Burdge says that in an omega-3 deficiency, omega-6 DPA accumulates in the plasma phospholipids and that the few studies of vegetarians that have reported omega-6 DPA levels have not found an accumulation. Burdge points out that Sanders and Reddy (1992) found no accumulation of omega-6 DPA in the red blood cells of infants breastfed by vegan mothers.
However, Burdge doesn’t include that Sanders and Reddy (1992) also found higher amounts of omega-6 DPA for Hindu vegetarians compared to omnivores in cord plasma (2.34% vs. 1.58%) and artery phospholipids (4.15% vs. 3.19%). These fractions weren’t reported for vegans.
The Hindu vegetarians had ALA intakes of only 0.9 g/day compared to 1.2 g/day for the vegans. While this difference isn’t much, 0.9 g/day is lower than the recommended DRI for omega-3s of 1.1 to 1.4 g/day and might be low enough that these Hindu vegetarians were deficient in omega-3s.
Burdge writes:
The findings of dietary supplementation trials and experiments using stable isotope tracers have been interpreted as indicating that humans are “poor” converters of [ALA], in particular with respect to DHA synthesis. The use of the terms “poor,” constrained or “limited”, [or] severely “restricted” to describe PUFA synthesis in humans presupposes that the level of conversion should be greater, but what this higher level should be has not been defined and is potentially misleading.
Rather, capacity for hepatic [ALA] conversion in humans is better considered as a product of an evolutionary history and, therefore, nutritionally adequate.
Conclusion to Evolutionary Arguments for a Dietary Requirement for DHA
The evolutionary arguments in favor of a dietary requirement for DHA are interesting but the evidence seems too vague to know whether they’re true. It appears that whether modern humans require dietary DHA for proper brain development and maintenance is a question that can only be answered through clinical trials comparing outcomes in people with and without a dietary source of DHA.
Appendix C: Stereodonic Acid
Stearidonic acid is a shorter-chain omega-3 that is unique because it does not require the rate-limiting enzyme that ALA requires to convert to EPA. There are very few plant-based foods that naturally contain SDA, specifically the oils of Echium plantagineum (echium) and Buglossoides arvensis (Ahiflower). There also are genetically modified soy and canola oils that are enriched with SDA.
Appendix D: Salvador, 2019
Salvador et al. (2019, Spain) studied 55 vegans and 49 lacto-ovo-vegetarians and found that those with a serum (not dietary) omega-6 to omega-3 ratio of ≤ 10 had a higher percentage of serum EPA and DHA than those with a ratio between 10 and 20 or >20 (EPA: 0.60%, 0.27%, and 0.23%; DHA: 2.90%, 1.91%, and 1.19% respectively). However, EPA and DHA were included in calculating the omega-6 to omega-3 ratio, while DPA wasn’t included.
Flaxseed intakes of once per day and, especially, 2 or more times per day were associated with a much higher percentage of serum ALA, but not with a higher EPA or DHA percentage; some subjects were taking EPA and DHA supplements.
This study sounds interesting because they did a number of analyses based on what they called the n-6/n-3 ratio, but it wasn’t the dietary short-chain n-6/n-3 ratio and the findings don’t appear to increase our knowledge of the issue.
Bibliography
Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids. 1995 Apr;30(4):365-9. (Abstract) Raw food vegans. Not cited.
Craddock JC, Neale EP, Probst YC, Peoples GE. Algal supplementation of vegetarian eating patterns improves plasma and serum docosahexaenoic acid concentrations and omega-3 indices: a systematic literature review. J Hum Nutr Diet. 2017 Dec;30(6):693-699. The six studies included in this review are all summarized in DHA Supplementation in Vegans. Not cited.
Crawford MA. Docosahexaenoic acid in neural signaling systems. Nutr Health. 2006;18(3):263-76.
Fisher M, Levine PH, Weiner B, Ockene IS, Johnson B, Johnson MH, Natale AM, Vaudreuil CH, Hoogasian J. The effect of vegetarian diets on plasma lipid and platelet levels. Arch Intern Med. 1986 Jun;146(6):1193-7. No omega-3 data. Found higher LA but similar AA levels among vegans, lacto-ovo-vegetarians, and meat-eaters. Not cited.
Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005 Apr;26 Suppl A:S70-5.
Kris-Etherton PM, Hill AM. N-3 fatty acids: food or supplements? J Am Diet Assoc. 2008 Jul;108(7):1125-30. No abstract available. Not cited.
Lands WE, Morris A, Libelt B. Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues. Lipids. 1990 Sep;25(9):505-16. Not cited above, but is citation #43 from Sarkadi-Nagy et al. (2004); mathematically complex paper which I couldn’t assess.
Li D, Sinclair A, Mann N, Turner A, Ball M, Kelly F, Abedin L, Wilson A. The association of diet and thrombotic risk factors in healthy male vegetarians and meat-eaters. Eur J Clin Nutr. 1999 Aug;53(8):612-9. Same measurements as provided in Li, 2002.
Li D, Turner A, Sinclair AJ. Relationship between platelet phospholipid FA and mean platelet volume in healthy men. Lipids. 2002 Sep;37(9):901-6. Same measurements as provided in Li, 1999.
Norris, J. Personal calculations. 2024. 1 teaspoon of dried chia seeds = 4 g (weighed on a food scale). USDA Nutrient database lists 28.35 g of dried chia seeds as having 5.05 g (5,050 mg) of ALA. 4 g * 5,050 mg ALA / 28.35 g = 712.52 mg ALA.
Peskin BS. Why fish oil fails: a comprehensive 21st century lipids-based physiologic analysis. J Lipids. 2014;2014:495761. (Retracted) Not cited.
Roshanai F, Sanders TA. Assessment of fatty acid intakes in vegans and omnivores. Hum Nutr Appl Nutr. 1984 Oct;38(5):345-54. Same study population as Sanders, 1992.
Sanders TA, Roshanai F. Platelet phospholipid fatty acid composition and function in vegans compared with age- and sex-matched omnivore controls. Eur J Clin Nutr. 1992 Nov;46(11):823-31. Same study population as Roshani, 1984.
Zhang Y, Jiao J. Reply to M Koch and MK Jensen. Am J Clin Nutr. 2016 Aug;104(2):537-8.

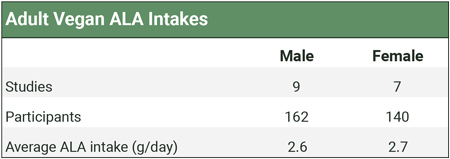
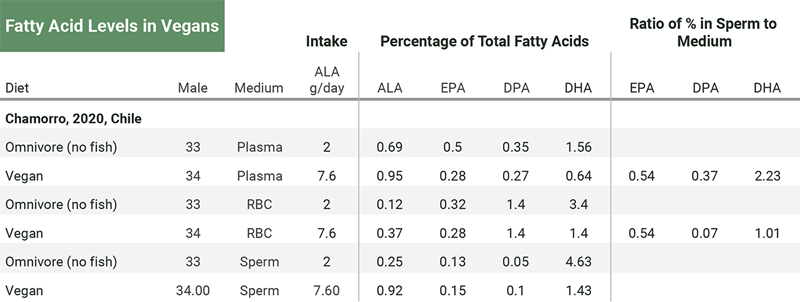
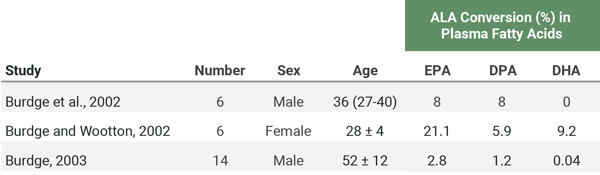
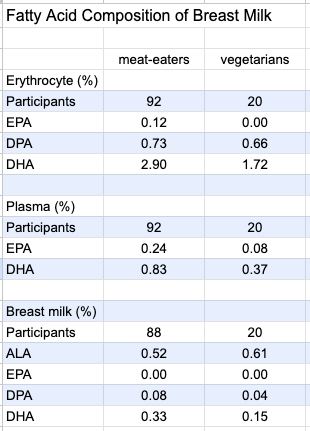
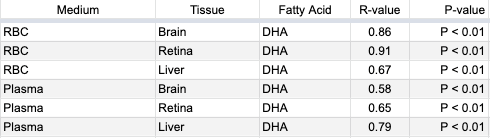
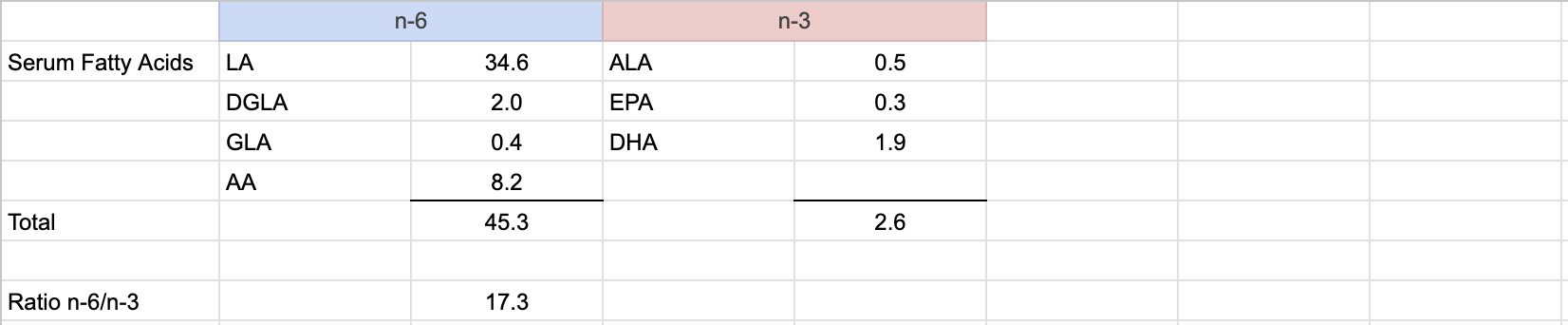
33 thoughts on “Omega-3s”
How many servings are in your Chia Seed Pudding Recipe? Thank you for your work as a vegan registered dietician and an animal activist. This website and
VeganOutreach.com are both tremendous resources for those of us who want to be vegan.
Mary,
Thank you for your kind words. According to my AI app, the chia pudding should yield 4 to 4.5 1/2-cup servings. That seems right if you add up the volumes of the ingredients. The servings would have about 150 to 200 calories depending on if you use sweetened milk and sweetener.
I wanted to suggest an addition to emphasize the endogenous enzyme constraints that can cap DHA production, even with high ALA intakes. As your page notes, ALA conversion to DHA is often down-regulated in those with adequate baseline levels [1], but studies further indicate that enzymes like delta-6 desaturase are rate-limiting and subject to saturation, where increasing ALA beyond a point yields minimal or no proportional DHA gains—sometimes even decreases due to feedback inhibition [2][3]. For instance, in a 12-week trial with high-ALA diets, ALA and EPA rose, but DHA declined in participants with low initial long-chain omega-3s [3]. This is exacerbated in males, where conversion to DHA is negligible (0-4%), compared to reproductive-age females [4][5]. High dietary LA, common in vegan diets, can suppress ALA conversion by 40-50% via enzyme competition [4][6].
I personally don’t believe any male can actually get healthy levels of Omega 3 long-term if we just rely on ALA and chia and flaxseed, nonetheless highlighting this information near the practical tips like the chia pudding recipe could help readers appreciate why direct algal DHA (200-300mg/day) might be preferable for many, especially men or those with high omega-6 intakes, to avoid suboptimal status [7][8]. This would enhance the page’s already strong guidance without detracting from ALA’s role.
Appreciate your commitment to accurate vegan health info—keep up the great work!
Best,
Martin
Links:
[2] https://pubmed.ncbi.nlm.nih.gov/12323085/
[3] https://pubmed.ncbi.nlm.nih.gov/9637947/
[4] https://pubmed.ncbi.nlm.nih.gov/16825676/
[5] https://pubmed.ncbi.nlm.nih.gov/12936959/
[6] https://pubmed.ncbi.nlm.nih.gov/17381964/
[7] https://pubmed.ncbi.nlm.nih.gov/28466678/
[8] https://pubmed.ncbi.nlm.nih.gov/25604340/
Martin,
Thank you for the rundown.
It looks like you forgot citation [1] and that you linked to the wrong abstract for citation [8]. I briefly looked at them and nothing seemed to suggest they measured EPA/DHA levels in tissues. Is that correct?
Citation [7], Canhada et al., seems like weak evidence for a general recommendation for long-chain omega-3 supplementation to prevent Alzheimer’s Disease.
Yes Jack, that is what I refer to about walnuts: mineral absorption because of antinutrients. Do walnuts need some kind of activation ? For example, what I do with almonds is to soak them for at least 9 hours, with a little of salt.
I wouldn’t worry about the antinutrient impact of a few walnuts a day. Our nutrient recommendations assume that people aren’t soaking, sprouting, or doing much of anything (except cooking foods that are normally cooked) to combat antinutrients. I would eat roasted almonds which should help decrease the antinutrient activity. A few daily, raw walnuts shouldn’t do much harm.
Jack, how are you. I have a doubt about Omega-3s. As far as I know, this fatty acid is easily damaged by heat, so people who eat fish they obviously have to cook it. What happens with Omega-3s in this case ? On the other hand, walnuts do contain antinutrients like legumes and other grains ? If so, is it necesary to activate them first ?
Martin,
I can’t find any studies looking at whether cooking fish oxidizes the omega-3s. Usually, oxidized oils smell rancid and cooking fish doesn’t seem to increase the rancidity of the smell, so that might be some very slim evidence that cooking doesn’t oxidize the oils. Antinutrients usually impact mineral absorption, not oils.
May I ask your source for the ALA content of chia seeds as 1 teaspoon = 713 mg? All the other sources I found (package labels and USDA database) show 1 TABLEspoon = 1.1g, which is about half that amount.
Michael,
I calculate that chia seeds have 713 mg per teaspoon according to the USDA database. I’ve added a citation to the table that points to this:
Norris, J. Personal calculations. 2024. 1 teaspoon of dried chia seeds = 4 g (weighed on a food scale). USDA Nutrient database lists 28.35 g of dried chia seeds as having 5.05 g (5,050 mg) of ALA. 4 g * 5,050 mg ALA / 28.35 g = 712.52 mg ALA.
– – – – – – – –
WHOLE
– – – – – – – –
Thank you for taking the time to measure. I can no longer find what I thought I saw in USDA and a package label which suggested half that amount. Today’s Dietitian does show a smaller amount of ALA per tsp, but I now see that I think they used the wrong weight conversion. I just measured 170g per cup which is close to USDA’s 168g / cup. Based on that:
USDA Food Central, Legacy Foods: 100g = 17.8g ALA
USDA Food Central, Survey Foods: 1C chia* = 168g (I measured 170g)
1C = 170g/100g x 17.8g ALA / 100g = 30.26g ALA / cup
1C = 48 tsp (168g ÷ 48 = 3.5g / tsp = 10.5g / Tbsp)
30.26g ALA ÷ 48 tsp = 0.63g = 630mg ALA / tsp
*Whole or ground not specified, which generally means whole, esp. b/c the only descriptor is “dried”.
That 630mg figure is pretty close to your 713mg figure.
Today’s Dietitian: 1.32g ALA ÷ Tbsp (= 440mg / tsp)
They say 1T = 7.4g, but from USDA, 1T = 10.5g.
After adjusting for 10.5g ÷ 7.4g x 440mg, we get 624mg / tsp.
– – – – – – – –
GROUND
– – – – – – – –
Many people will use ground chia since the ALA is much better absorbed that way. I calculate that as follows:
1C ground chia = 108g†
108g x 17.8g ALA / 100g = 19.2g ALA
19.2g ALA / 48tsp = 0.4g = 400mg / tsp
† From my measurement. I was surprised that it weighs less than whole seed, not more, but it did get pretty fluffy from grinding.
That’s 108g ÷ 48 tsp = 2.25g chia/tsp, which is pretty close to Spectrum Organics’ label.
Central Market’s label says 12g / 1T = 4g / tsp, which differs significantly.
–> Too much omega-3 can result in bleeding and bruising.
Bingo. This is probably the reason why it happens to me recently. I am meeting easily RDA of ALA (and more than RDA), but recently added algae DHA/EPA supplement.
Do you have an opinion about the possible concerns in relation to the high omega-6 content of tofu? The ideal ratio of omega-3 to omega-6 in food is 1-1 to 1-4. A small portion of 100 grams of tofu contains about 3.8 grams of omega-6 per 100 grams. You can then rebalance the ratio back by taking the same amount of omega-3 algae oil as a supplement. But firstly, tofu portions are often larger than 100 grams, secondly, other foods also contain omega-6 (a handful of mixed nuts already contains 5 grams of omega-6, a glass of soy milk 2-4 grams), thirdly, omega-3 supplements are expensive, and fourthly, there is a maximum to the number of omega-3 supplements you can take per day to restore the balance (max dosage per day, I think, is about 3 grams). What can you share about the best way to optimize protein intake while balancing omega-3/ omega-6 on a healthy vegan diet for optimum long term health?
Alexander,
At this time, I have no concerns about eating typical amounts of foods that are high in LA. Your question inspired me to clarify what I’m saying about LA to ALA ratios in this article. Because LA to ALA ratios were considered to be important for a long time, I was paying more lip service to the subject than I probably should have been. While I don’t think there’s conclusive evidence that the ratios don’t matter, I definitely lean toward the view that they don’t matter based on a lack of evidence.
Hi, I was wondering whether there is any guidance relating to the best time to take algal EPA/DHA supplements? i.e with meals or particular times of day? I’m aware that increased amounts of LA interfere with ALA absorption, however, does taking an EPA/DHA supplement alongside a meal rich in omega 6’s and 9 interfere with the absorption of the omega-3’s from the supplement?
Alison,
I’m not aware of research on the timing of taking algal EPA/DHA. I looked at one randomized controlled trial of vegans supplementing (Sarter, 2015) and they didn’t instruct the participants on when to take the supplement.
> I’m aware that increased amounts of LA interfere with ALA absorption
LA can interfere with the conversion of ALA to EPA and DHA but I’m not aware of it interfering with absorption. Also, LA interfering with conversion has only been shown in blood; it hasn’t been studied in human tissues and so the physiological significance is questionable.
You might be interested in a new review of ALA intake on cardiovascular and cancer mortality.
Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O et al. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies BMJ 2021; 375 :n2213.
The results found that ALA intake decreased cardiovascular disease but slightly raised cancer mortality risk. I have read your post “ALA and Prostate Cancer Update” but I would be interested to know what you think about these results from the 2021 review.
Darryl,
According to the abstract, the relative risk for cancer was only 1.06 and barely reached statistical significance, so I’d be surprised if it wasn’t due to confounding. Personally, it doesn’t give me any pause with regard to eating a few grams per day of ALA.
ALA and LA compete for the same rate-limiting enzyme. But that doesn’t mean that lower LA and higher ALA intake can improve DHA or EPA status in any way. ALA is definitely stored in the body and even if there is too little enzyme, ALA can be converted later. Am I right? I’m not a biologist so I can be very wrong.
N,
There’s evidence that lower LA and higher ALA intakes can result in a higher conversion of ALA to long-chain omega-3 fatty acids. It’s not clear to what extent the ratio impacts the conversion.
Hello. The article claims that “in cases of very low maternal levels of DHA, infants can utilize other fatty acids for brain tissue which can later be replaced by DHA” and the article you mentioned (32) doesn’t say that directly. Is there any information about this? Thank you!
N,
The paper by Langdon says, “An overall body deficiency permits DHA to be substituted by other fatty acids within the membranes, including docosapentaenoic acid (22: 5n-6) or arachidonic acid (20: 4n-6), so that body tissue composition can shift fairly quickly as diet changes (Neuringer et al. 1988; Uauy, 1990; Neuringer, 1993). This replacement may be temporary, until body levels of DHA are restored, and it implies that temporary shortages of DHA need not interfere with the rate of overall brain growth.”
Where Langdon says, “within the membranes,” I’ve assumed he’s referring to nerve cell membranes in the brain, but perhaps he’s referring to membranes in other types of cells shifting DHA to the brain. I’ll try to contact him and find out.
N,
Dr. Langdon replied to my inquiry in minutes, saying, “My understanding is this refers to cell membranes within the central nervous system.” Based on his response, I think my interpretation is accurate, though I suppose we could quibble about whether “central nervous tissue” necessarily includes “central nervous tissue within the brain.” What do you think?
I’m not good at biology but Internet says Central Nervous System does include brain.
Your website is absolutely amazing, I appreciate very much all the work here.
N,
Yes, I just meant that while brain tissue is in the central nervous system, it’s still possible to differentiate between nervous tissue in the brain part of the central nervous systems vs. the non-brain part of the central nervous system. Looking into Landon’s references would probably clarify this.
Thanks for your kind words about the site!
It took me a while, but I tracked down Langdon’s references and I don’t believe his view is justified. I’ll soon be updating the page to reflect what I’ve found and sending out a blog post (subscribe in the footer of this page). I’ve taken out the line about Langdon since I no longer consider it justified.
Hi,
Given concerns about oxidation reducing the efficacy of omega 3 supplements (and even possibly making them harmful), would you still recommend taking an omega 3 supplement? If one thinks they could increase ALA intake instead from non-supplement food sources, would you recommend that in place of a supplement? Here’s a source for the concerns I mentioned:
https://www.hindawi.com/journals/bmri/2013/464921
Thanks,
Sean
Sean,
The potential oxidation level of long-chain omega-3 fatty acid supplements is concerning, but as Albert et al. point out in the article you link to, Ottestad et al. didn’t find a negative impact of oxidized fish oils over a 7-week period.
We don’t necessarily recommend that vegans take an EPA/DHA supplement; our recommendations are to meet the RDA for ALA (which, unfortunately, some vegans fall short of), and for people who want an extra measure of safety, we recommend obtaining additional ALA or taking a 200-300 mg DHA supplement per day.
As research continues to show long-chain omega-3 supplements to have, at best, only weak associations at preventing heart disease, dementia, and depression, the supplement option seems less necessary. The one concern I continue to have is that vegans’ blood levels of DHA are typically quite a bit lower than meat-eaters; but as we learn more, my sense is that this isn’t a reflection of the body’s lack of DHA in nerve tissue.
“recommendations beyond the dietary reference intake for ALA (see Daily Needs)” =>.”additional 2,000 mg”
A poor amount to recommend, given that :
“3.7 g of ALA… didn’t significantly increase the percentages of EPA or DHA.”
X,
The response I gave to N below is also my response to your point.
According to the listed tables, I doubt that supplementing additional 2g of ALA will be enough. Increasing ALA consumption does not increase DHA level therefore I, as a new vegan, may experience DHA deficiency in the future. I can’t afford DHA supplies but flax seeds aren’t scarce at all.
N,
I don’t think it’s necessary for most vegans to supplement with DHA for a few reasons:
1. As mentioned above, a 1992 study in India of 5 vegetarians found a 1/3 increase in the absolute amount of DHA in plasma phospholipids after 6 weeks of 3.7 g/day of ALA (Ghafoorunissa, 1992). This is the only study I’m aware of that measured absolute amounts (rather than percentages), and without evidence to the contrary absolute amounts seem more important than percentages to me.
2. A 2014 study found that vegan men had similar DHA levels to a segment of men who didn’t eat fish. A 2020 meta-analysis of six cohort studies found no correlation between eating fish and a reduced risk of cardiovascular disease or mortality (Zhong, 2020)
3. We don’t know what a healthy level of DHA is and it seems likely that there are feedback loops that prevent ALA conversion once an optimal level is reached, so many of the studies measuring ALA conversion might show no improvement because DHA needs are already being met.
4. DHA in the blood might have little correlation with DHA in tissues.
5. We don’t see any impact among vegans who don’t supplement with DHA that can be reasonably attributed to poor DHA status.
Thank you very much. Yes, I learned much about DHA. There is a study that shows that higher ALA intake actually increases DHA level. I don’t supplement and I feel OK.