
by Jack Norris, RD
Contents
- Summary
- Introduction
- Plant Foods with Practically No Detectable B12 Analogue
- Fermented Foods
- Mankai (Duckweed)
- Algae
- Seaweeds (Macroalgae)
- Various Seaweeds: Dulse Warrants Further Study
- Nori
- Vitamin B12 Analogue Content of Nori Species
- Nori (Neopyropia sp.) Lowers Serum MMA Levels among Vegetarians (2024)
- Nori (P. tenera) Fails to Lower Urinary MMA Levels (1999)
- Coccolithophorid Algae
- Ulva Fenestrata
- A Case of False Reporting on the Benefit of Seaweed and Fermented Foods
- Genmai-Saishoku Paradox?
- German Whole Foods Vegans Consuming Nori and Mushrooms
- Soil and Organic Produce as a B12 Source for Vegans
- Bibliography
Summary
In the published research, one plant food, chlorella, has been shown to have vitamin B12 activity in humans; there are caveats that you should be aware of before relying on it (see below). The only other plant food that has been tested is nori, which did not have B12 activity.
A number of foods, arguably, warrant further attention. Makai, a type of duckweed, has shown promise for containing active B12 due to synergistic bacteria living inside the plant. But unless these foods are shown to consistently improve B12 status, vegans should not rely on them for vitamin B12.
Introduction
It could be a boon to the vegan movement to find a source of vitamin B12 that naturally and reliably exists in a vegan food. In their zeal to find such a source, some vegan advocates recommend foods whose ability to provide vitamin B12 is sketchy at best. Because of the harm that can come to someone relying on such foods for vitamin B12, I review the published scientific research below with a skeptical view.
There has been a long history of misconceptions about which, if any, plant foods are sources of B12. Much of this stems from the methods of measuring B12 analogues. Other confusion stems from bacterial contamination that occurs in some foods but not others. Please see Measuring B12 in Plant Foods: Why the Confusion? for an explanation of the methods for for measuring B12 analogues in plant foods.
Unlike animals, most, if not all, plants have no B12 requirement for any function, and therefore have no active mechanisms to produce or store B12. When B12 is found in them it can be due to contamination which is not reliable.
Many seaweeds have been shown to have B12 analogues. Most seaweeds are macroalgae, which are technically not plants. Some macroalgae contain an enzyme that can use cobalamin, but also have an enzyme with the same function that does not require cobalamin in case it is not present. These macroalgae do not make their own cobalamin, but rather have a symbiotic relationship with cobalamin-producing bacteria (Smith, 2007). Note that I am purposefully using the term “cobalamin” rather than “vitamin B12” because it is not clear if these cobalamins are active vitamin B12 in humans.
During the 1970s, two enzymes in plants (potatoes and bean seedlings) were found to respond to the addition of adenosylcobalamin (Poston, 1978, Poston, 1977), a co-enzyme form of B12. One explanation is that adenosylcobalamin provides some factor that is usable by these enzymes, but that adenosylcobalamin is not required by these plants for growth. Thus far, these plants have not been shown to counteract B12 deficiency symptoms (though I am not aware of any well-designed attempts as it is assumed that they do not contain B12). It is probably safe to assume that many vegans who have developed severe B12 deficiency ate potatoes and beans.
There are some rumors, though no evidence of which I am aware, that if you let organic produce, such as carrots, sit at room temperature for a few hours, bacteria on the surface of the carrots will produce B12. For this to happen, specific species of bacteria would be required, as would cobalt, on the surface of these foods. Until there is research showing that such a method can lower MMA levels, such produce should not be considered to provide B12.
Many of the studies below analyze the vitamin B12 analogues in various foods to determine if the food contains vitamin B12, rather than feeding various batches obtained in typical food markets, to people to see if it improves their vitamin B12 status. There are significant problems with this approach because:
- Even if you find some molecules that seem to be vitamin B12, you don’t know how it will interact with other inactive B12 molecules inevitably also prevalent in these foods.
- We do not know how the B12 got there: whether the plant made it (unlikely), whether it has come from symbiotic bacteria, or whether it came from fecal or insect contamination. Thus, we do not know how reliable it would be in other batches of that food throughout the world.
- The packaging, storage, transportation, and preparation methods can differ greatly between the careful laboratory methods used in these reports and the versions someone might buy in a grocery store.
It cannot be emphasized enough that until a particular food, obtained from multiple regions, consistently improves vitamin B12 status (via lowering MMA levels), it should not be relied upon as a source of vitamin B12.
Plant Foods with Practically No Detectable B12 Analogue
Various studies have tested the foods in Table 1 for B12 analogues and found none. To my knowledge, other than in studies (described below) in which B12 or cow manure were carefully added to the growing medium of plants, no published study has shown any B12 analogues in any of these foods.
| Table 1. Foods with No Detectable B12 Analogue |
|---|
| Amesake riceA |
| Barley misoA |
| MisoB |
| NattoB |
| Rice misoA |
| ShoyuA |
| TamariA |
| Umeboshi prunesA |
| Various fruits, vegetables, nuts, seeds, & grainsB |
| A. Van den Berg, 1988 B. Areekul, 1988 |
Table 2 shows the B12 analogue content of various plant foods.
| Table 2. B12 Analogue Content (mcg/30 g) of Various Foods | ||
|---|---|---|
| NetherlandsA | ThailandB, C | |
| Assay | Intrinsic Factor (IF) | IF or R-protein |
| Fermented soybean | 0.15 | |
| Barley malt syrup Sourdough bread Parsley Shiitake mushrooms |
.006-0.1 | |
| Dried fermented soybean | 0.01 | |
| Tofu | None Detected | 0.02 |
| Soybean paste | 0.03 | |
| Soy sauce | .01 µg/30 ml | |
| A. Van den Berg, 1988 B. Areekul, 1988 C. Areekul, 1990 |
||
As you can see, there are very small amounts, if any. Since the amounts are so small, any inactive analogs should not significantly interfere with an individual’s active B12 from other sources, and if the analog is active B12, it will not provide much. Thus, these foods should neither add to, nor detract from, a vegan’s B12 status.
Fermented Foods
Because bacteria produce vitamin B12 and fermented foods are generally fermented using bacteria, there are many rumors regarding vitamin B12 being in fermented foods. To my knowledge, no vitamin B12-producing bacteria is required for any fermented food and, therefore, any fermented food that contains vitamin B12 does so via contamination. Because the human colon contains vitamin B12-producing bacteria, it is possible for B12-producing bacterial contamination to occur during food preparation, particularly in places that do not have high levels of cleanliness. To my knowledge, no fermented plant food in Western countries has been found to contain relevant amounts of vitamin B12 analogues.
Tempeh
While tempeh is usually made by fermenting soybeans, it can also be made using a different species of legume, lupine beans. The fungus used to create lupine tempeh is Rhizopus oligosporus, which doesn’t produce B12. However, Signorini et al. (2018) added a B12-producing bacteria, Propionibacterium freudenreichii, to the tempeh fermentation process resulting in 1.2 µg of B12 per 100 g dry weight of the lupine tempeh. The study didn’t address the costs of producing B12 in this way compared to simply producing supplements, but this fermentation process holds promise as a way to provide a source of B12 in plant foods.
Table 3 shows the B12 analog content of various soy tempehs in which a known B12-producing bacteria wasn’t purposefully added to the fermentation process.
| Table 3. B12 Analogue Content (mcg/30 g) of Tempehs | |||
|---|---|---|---|
| NetherlandsD | USAE | IndonesiaF,G | |
| Assay | Intrinsic Factor | Intrinsic Factor | Intrinsic Factor or R-proteinA |
| Tempeh | None | .02C | .054-1.2B |
| A. Used an assay method by Lau, 1965 which uses R-protein or IF B. 10 commercial tempeh samples purchased from various markets in Jakarta, Indonesia C. Cooked for 60 minutes D. Van den Berg, 1988 E. Specker, 1988 F. Areekul, 1988 G. Areekul, 1990 |
|||
The studies in the USA and in The Netherlands showed little to no B12 analog.
In contrast, Areekul et al. (Areekul, 1990) (Indonesia/Thailand) found more significant amounts of B12 analogs. Tempeh production requires molds belonging to the genus Rhizopus. These were found not to produce B12 analogs in Areekul et al.’s study. Rather, a bacterium, identified as Klebsiella pneumoniae, was isolated from the commercial tempeh starter and determined to be the B12 analog source. This confirmed Albert et al.’s (Albert, 1980) finding that the Klebsiella genera could produce B12 analogs. In Albert’s study, the analog was thought to be active B12. Whether the analogs found by Areekul et al. were the same as in Albert’s study is not known. Given that K. pneumoniae is not required for tempeh production, we can conclude that the B12 analog found in the tempehs in Indonesia were due to bacterial contamination (though apparently common there). Tempeh in Europe and the U.S. cannot be relied on as a source of B12. Until tempeh in Indonesia is shown to reduce MMA levels, it should not be relied upon there, either.
Japanese fermented black tea (Batabata-cha)
A 2004 study by the Watanabe group found that fermented black tea (Batabata-cha) contained vitamin B12 analogs that, when fed to rats, improved their vitamin B12 status (Kittaka-Katsura, 2004). It would be interesting to see if this tea could consistently improve B12 status in humans.
Korean Centenarians
A 2010 paper from Korea (Kwak, 2011) showed that Korean centenarians (people who live to be 100 years old) who ate only small amounts of animal products had normal vitamin B12 levels. The researchers measured the B12 content of plant foods using a biological assay and found many of the fermented foods and seaweeds to contain vitamin B12 analogues, which they considered to be active. They determined that the centenarians were getting about 30% of their B12 from plant foods and that it was a physiologically important amount.
This could be the case, especially given that the subjects ate fermented foods at almost every meal, much of which is homemade kimchi that, according to the researchers, is fermented for at least 10 months.
While this study is very interesting, unless kimchi produced in western countries is reliably shown to lower MMA levels, it would not be wise to rely on it as a significant source of vitamin B12.
Lactobacillus species
Lactobacillus is a genus of bacteria found in some people’s digestive tracts and in most probiotic supplements. There is evidence that some species produce vitamin B12.
A 2003 study of Lactobacillus reuteri CRL1098 determined that it produces vitamin B12 and that this B12 was equivalent to cyanocobalamin (Taranto, 2003).
In a 2006 study from Egypt, school children were fed yogurt fermented only with Lactobaccillus acidophilus, 2 cups daily with 5 X 109 colony-forming units (Mohammad, 2006). After 42 days, their B12 status was compared to children who were fed a commercially prepared yogurt. Urinary MMA levels went from 3.49 to 2.09 mmol/mol of creatinine in the experimental group (P = .02) versus no change in the commercial yogurt group.
In a 2000 study of vegan raw foodists, 4 vegans were fed a probiotic supplement containing Lactobacillus acidolphilus and other Lactobacillus species (Donaldson, 2000). After 3 months, the urinary MMA levels of 3 of the 4 subjects had decreased, though not to normal levels. More details of this study are on the page, Raw Foodist Vegans.
While Lactobacillus shows some promise, it is too soon to rely on it for keeping your vitamin B12 status at healthy levels.
Mankai (Duckweed)
Wolffia globosa is commonly known as Mankai and is a type of duckweed. A group of researchers has been examining whether Mankai can serve as a plant-based source of B12. They believe that the B12 is produced by bacteria living inside the plant tissue, known as endophytic bacteria.
Their 2019 study reported that a cutlet made from Mankai duckweed (a specific strain of Wolffia globosa, an aquatic plant) contained 2.8 µg of B12 per serving (Kaplan, 2019). The food wasn’t tested for overall B12 activity which is always necessary to determine if the B12 found in a food is both active for humans and that no inactive B12 analogs are interfering with its activity.
In a subsequent report, the researchers test Mankai for B12 in three different ways (Sela, 2020):
DIRECT PLUS Dietary Intervention Trial
The researchers measured changes in serum B12 during an 18-month, weight-loss clinical trial in which participants took part in a workout program and were divided into three dietary interventions:
- Healthy dietary guidelines (HDG) group: Participants received basic healthy diet guidelines.
- Mediterranean (MED) group: Participants were instructed to adopt a calorie-restricted Mediterranean diet.
- Green Mediterranean (Green-MED) group: Participants were instructed to follow the MED diet, avoid red and processed meat, and to consume 3–4 cups/day of 100 g frozen cubes of Mankai in a green shake. The researchers thought 100 g of Mankai should contain about .5 µg of B12.
After 18 months, serum B12 levels increased an average of 5.2% in the HDG group (n=92), 9.9% in the MED group (n=84), and 15.4% in the Green-MED group (n=89). The difference in net changes between groups was statistically significant.
The B12 intake of the diet groups wasn’t assessed. Both the MED and Green-MED groups increased their milk and egg consumption, and the Green-Med group also increased fish consumption making it impossible to tell if the increase in serum B12 levels was from animal foods or from Mankai. Another important caveat is that unless you’re measuring methylmalonic acid levels, it’s difficult to assess a food’s impact on B12 status.
Bioassay and Liquid Chromatography
Using both a bioassay and liquid chromatography, the analysis of Mankai samples indicated that, as far as the tests could determine, the B12 analogs in Mankai were structurally equivalent to active B12 in humans. The researchers took precautions to avoid bacterial contamination of the Mankai from external sources.
Exposing Human Fecal Bacteria to Mankai
The researchers exposed human fecal bacteria, in vitro, to Mankai to see if the population of B12-dependent bacteria increased. Mankai-supplemented samples displayed significantly more gene sequences associated with B12 uptake than did control samples lacking Mankai.
These three methods for assessing whether Mankai contains active vitamin B12 all had promising results. In our January 2020 post, Is Duckweed a Source of Vitamin B12?, we expressed skepticism that the synergistic bacteria strains that establish themselves within Mankai would randomly also be bacteria that produce active B12 for humans. This latest report lends more credence to the possibility.
What we’d like to see is Mankai consumed by people, who consume no other source of B12, over a period of a few months to determine the impact on their methylmalonic acid levels. If Mankai has a significant, positive impact reasonably equal to B12-fortified foods, then it would be worth duplicating the study with Mankai from another region and in a different laboratory. If the study’s results were replicated, we’d be able to at least begin considering Mankai as a reliable source of B12 for vegans. Until then, it wouldn’t be prudent.
Algae
Blue-green algae are also known as cyanobacteria, blue-green bacteria, and cyanophyta. They are not actually algae, but rather organisms with characteristics of both bacteria and algae. They can perform photosynthesis and are thought to be the ancestors to chloroplasts in algae and plants.
Aphanizomenon Flos-aquae
Some companies have marketed algae from Klamanth Lake in Oregon. Cell Tech was one of the most prominent seller’s of such algae for many years. They used a the strain, Aphanizomenon flos-aquae, which they called Super Blue Green Algae (SBGA) and sold via a multi-level marketing plan. On April 16, 2003, Cell Tech’s now defunct website stated:
“Is the vitamin B12 in SBGA bioavailable and bioactive? Yes. The Super Blue Green Algae (SBGA) strain, Aphanizomenon flos-aquae, has been tested by Lancaster Labs for B12 analog levels using microbiological testing methods that are comparable to methods 952.20 and 960.46 of the Association of Analytical Chemists (AOAC). Unlike other plant foods such as Spirulina, which contain corrinoids with virtually no vitamin B12 activity, Aphanizomenon flos-aquae is a reliable source for vegetarians seeking to supplement their diets with a bioactive form of this important nutrient.”
However, test methods 952.20 and 960.46 use Lactobacillus leichmannii (Helrich, 1990), which can measure non-B12 corrinoids (Schneider, 1987). See the Microbiological Assay in Measuring B12: Why the Confusion? Thus, it can only be concluded that Cell Tech’s SBGA contains B12 analogues whose activity is yet to be determined.
2010 Update: It appears that Cell Tech is now the company, Simplexity Health, and is no longer touting SBGA as a source of vitamin B12.
In a 2009 study from Italy (Baroni, 2009), researchers gave Aphanizomenon flos-aquae to 15 vegans. First there was a washout period in which the vegans took no supplemental B12 for 3 months. They were then given 6 capsules of Klamanth Algae from Nutratec (which also contained digestive enzymes to help absorption).
The results, seen in Table 4, show that the average homocysteine level went down. The authors believe this is an indication that Aphanizomenon flos-aquae is a source of active vitamin B12, and that it “warrants further larger, and longer-term randomized trials to confirm such preliminary conclusions.”
| Table 4. Supplementation with Aphanizomenon flos-aquae | |||
|---|---|---|---|
| Marker | Baseline | 3 mosA | 6 mosB |
| Homocysteine (µmol/l) | 13.7 | 15.2^ | 12.0* |
| Serum B12 (pg/ml) | 259 | 196^ | 237 |
| Folate (ng/ml) | 11.0 | 10.9 | 12.5 |
| ^Statistically significant difference from baseline. *Statistically significant difference from 3 months. |
|||
Here are some problems with the study:
- The authors state in the paper that homocysteine is the most reliable marker for B12 activity, but it is not. Homocysteine levels can be affected by folate intake and, to a lesser extent, vitamin B6. Methylmalonic acid levels are the most reliable marker for B12 activity. This is well known and uncontroversial, so it is odd that the researchers did not know this.
- The authors noted that vitamin B6 could not have reduced the homocysteine levels because the algae has very little. They also said that folate levels could not have affected them, but in looking at the results, folate levels did increase (even though the difference was not statistically signifcant).
- The homocysteine levels of these vegans started out pretty high, and when the study ended they were still much too high. A safer level is closer to 6-8 µmol/l.
- One subject’s homocysteine level increased, and one subject’s homocysteine level that was about 10 µmol/l did not respond to the aglae supplementation.
- The researchers obtained the algae directly from a company that produces it. It would have been more reassuring if the algae were purchased in a store where the company didn’t realize it was going to be tested.
In another study from Italy (2002) (Bissoli, 2002), vegetarians had really high homocysteine levels (25 µmol/l). This is much higher than almost all other studies, which makes one wonder what’s going on in Italy.
In conclusion, it appears that Aphanizomenon flos-aquae might provide some vitamin B12 activity in humans. On the other hand, it did not succeed in lowering homocysteine to an ideal level whereas vitamin B12 supplements do succeed at doing so. At this time, it would be prudent not to rely on it for optimal health.
Chlorella
A 2015 study found an increase in B12 levels and a decrease in MMA levels in vegetarians and vegans taking chlorella over the course of 60 days. More research is needed before chlorella can be recommended as a reliable source of B12.
2015 Clinical Trial Using Chlorella
A 2015 USA study fed 17 vitamin B12-deficient vegans and vegetarians a Chlorella pyrenoidosa supplement for 60 days (Merchant, 2015). The study used 3 doses of Sun Chlorella A tablets per day, taken with meals, for a total of 9 g of chlorella per day. Based on a 2002 study by Kittaka-Katsura et al., they believed that 9 g of chlorella contained 21 µg of B12 (see table below).
| B12 Analog Content of Chlorella | ||
|---|---|---|
| Detection Method (µg B12 per 30 g chlorella) |
||
| Lactobacillus leichmannii | Intrinsic factor chemiluminescence | |
| Manufacturer A | 72 | 60 |
| Manufacturer B | 86 | 63 |
| Manufacturer C | 60 | 62 | Source: Kittaka-Katsura, 2002. Chlorella samped from a local market in Kochi-City, Japan. |
Average serum MMA levels decreased from 441 nmol/l at baseline to 301 nmol/l at 30 days and to 297 nmol/l at 60 days. Normal MMA is typically defined as less than 270 nmol/l, although 297 nmol/l is arguably also healthy (see Minimizing Methylmalonic Acid Levels). Average serum homocysteine levels decreased from 10.0 µm/l at baseline to 9.5 µmol/l at 30 days and 9.0 µmol/l at 60 days. These changes reflect a practical amount of B12 activity.
A confounding variable could be the B12 from animal products that the vegetarians in the study were eating. The B12 intakes of the participants weren’t measured before or during the study, but the subjects were asked not to change their diets or supplement regimens, so the changes in MMA levels should reflect an impact of the chlorella. No adverse effects were noted from the chlorella regimen. The study was funded by the Sun Chlorella Corporation of Japan, and the lead author of the study is a paid consultant by the company.
The participants took a total of 45 tablets per day, which for most people would be an expensive regimen. As of November 2020, 45 tablets containing a total of 9 g of chlorella and 21 µg of B12 (according to their label) would cost about $4.00 per day (see spreadsheet B12 in Chlorella). However, 3 daily servings of chlorella containing 7 µg of B12 each might not be necessary for maintaining B12 levels. For a regimen of 3 doses of cyanocobalamin per day, we recommend at most 1.3 µg of B12 per day for adults which would be about $25 per month of Sun Chlorella A; for 2 doses per day, we recommend 5 µg which would cost about $30 per month.
The significant drawback of relying on chlorella for B12 is that chlorella doesn’t produce the B12; rather, it absorbs it from its environment, likely due to bacterial contamination (Kittaka-Katsura, 2002). This makes it especially important either that a) batches from a wide variety of sources are consistently shown to contain B12, or b) that a particular company is able to understand and replicate how their chlorella absorbs B12 from the environment.
Other Research on B12 in Chlorella
Using capillary electrophoresis, a Taiwanese study found cyanocobalamin in two samples of chlorella purchased locally (Chen, 2008). Capillary electrophoresis should be able to detect the exact structure of a cobalamin analog. They found negligible amounts of inactive B12 analogs.
In the Autumn 2005 issue of their newsletter, The Vegan (p. 30), the UK Vegan Society reported on a trial they performed using chlorella and spirulina to treat elevated MMA levels. While they considered the trail “inconclusive” the one person who stayed in the trial and supplemented with chlorella did see a normalization of MMA levels. The article doesn’t provide details such as the length of the trial, the amount of chlorella, or the MMA levels.
A 1968 USA study analyzed numerous batches of Chlorella vulgaris and Chlorella pyrenoidosa using Euglenis gracilis and Ochromonas malhamensis bacteria cultures (Pratt, 1968). They found very little B12 and suggested that what they did find could be due to bacterial contamination of their samples. They hypothesized that the cell walls of the chlorella might have prevented the release of B12, among other possibilities limiting the detection of B12.
Spirulina
An Indian research group published an article in 2010 examining the vitamin B12 content of spirulina (Spirulina platensis). They believed that they found 35 – 38 µg of methylcobalamin per 100 g of dry mass (Kumudha, 2010).
Table 5 shows the B12 analogue content (µg/30 g) of various spirulina batches from earlier reports:
| Table 5. B12 Analogue Content (mcg/30 g) of Spirulina | |||||||
|---|---|---|---|---|---|---|---|
| NetherlandsA | USAB | JapanC | |||||
| Assay | IF | L. leich. | IF | L. leich. | L. leich. | IF | PC |
| Spirulina | 14.5 | 67 | 36.7 | 193.1 | 73 | 2.5 | 0.44 |
| Spirulina | 6 | 35.3 | 38 | 1.9 | 0.32 | ||
| Spirulina | 1.67 | 8.7 | 44 | 5.2 | 0.88 | ||
|
A – Van den Berg, 1988 B – Herbert, 1982 C – Watanabe, Katsura et al., 1999 IF – Intrinsic factor Assay PC – Paper Chromotography Assay |
|||||||
The wide range of B12 analogues from one measurement method to another indicates that spirulina has a wide variety of different analogues, many of which are inactive. Some may interfere with B12 activity in humans.
In the one study published in medical journals testing spirulina, B12 activity actually decreased in people fed a combination of spirulina and nori (Dagnelie et al., 1991, Netherlands).
In the Autumn 2005 issue of their newsletter The Vegan (p. 30) the UK Vegan Society reported on a trial they performed using chlorella and spirulina to treat elevated MMA levels. Three people with abnormal MMA levels were given spirulina and their MMA levels remained abnormal.
Suizenji-nori
Watanabe et al (2006, Watanabe, 2006) found only what they considered to be inactive vitamin B12 analogues in the blue-green algae, Suizenji-nori.
Seaweeds (Macroalgae)
Various Seaweeds: Dulse Warrants Further Study
Table 6 shows the B12 analogue content of arame, dulse, hijiki, kelp, kombu, and wakame per 30 g of seaweed. Please note that 30 g is a lot of seaweed. A serving size would be closer to 3 grams. Seaweeds also tend to be very high in iodine, which can cause problems at high intakes. So, consuming mass quantities of seaweed is inadvisable.
| Table 6. B12 Analogue Content (mcg/30 g) of Various Seaweeds | |||
|---|---|---|---|
| NetherlandsC | USAD | ||
| Assay | IF | L. leich. | IF |
| Arame | 0.042 | ||
| Dulse (Palmaria palmata) | 3.9 | 3 | |
| Hijiki | < .006 | < .006 | |
| Kelp | 1.2 | 0.12 | |
| Kombu | 0.84 | 0.018 | .57-1.3A |
| Wakame | 1.4 | 0.009 | 1.29B |
| IF – Intrinsic factor Assay A – Range of 5 samples of 3 different brands, with 3 samples cooked for 60 minutes B – Cooked for 60 minutes C – Van den Berg, 1988 D – Specker, 1988 |
|||
The only seaweed in this list that warrants further study is dulse (also spelled “dulce”), which contains .3 to .39 µg of B12 analogue per 3 g serving. Unless dulse is eventually shown to lower MMA levels, it should not be considered a source of active B12.
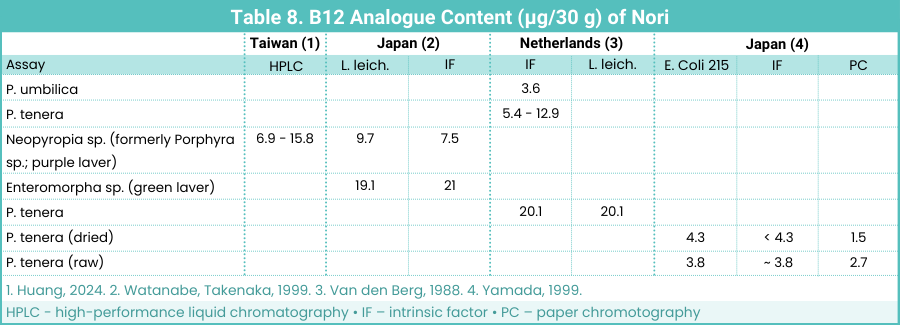
Nori
Nori is used in many countries for wrapping sushi. The term nori typically refers to purple laver belonging to the species Neopyropia sp., formerly known as Porphyra sp. (Huang, 2024). Nori can also refer to the genus Enteromorpha, which is a green laver. While we’ve traditionally talked about different species as nori, it’s probably not appropriate to discuss different species of nori as though they’re the same, or even related, foods.
One sheet of Ocean’s Halo Organic Sushi Nori weighs 2.8 g and makes about 6 normal-sized sushi rolls as consumed in the United States.
Vitamin B12 Analogue Content of Nori Species
Table 8 below shows the B12 analogue content of various nori types and batches:
Batches of nori were found to contain significant amounts of B12 analogue. Yamada et al. (1996, Japan) determined that nori contains what they considered to be active B12 analogues using various assays and methods (results not reported here).
Other studies have measured the B12 analogue content of nori, but without testing to see if it could lower MMA levels:
- Watanabe F, Takenaka S, Katsura H, Miyamoto E, Abe K, Tamura Y, Nakatsuka T, Nakano Y. Characterization of a vitamin B12 compound in the edible purple laver, Porphyra yezoensis. Biosci Biotechnol Biochem. 2000 Dec;64(12):2712-5. (Abstract)
- Miyamoto E, Yabuta Y, Kwak CS, Enomoto T, Watanabe F. Characterization of vitamin B12 compounds from Korean purple laver (Porphyra sp.) products. J Agric Food Chem. 2009 Apr 8;57(7):2793-6.
- Kwak CS, Hwang JY, Watanabe F, Park SC. Vitamin B12 Contents in Some Korean Fermented Foods and Edible Seaweeds. Korean J Nutr. 2008 Jul;41(5):439-447. (Abstract) Article in Korean.
Nori (Neopyropia sp.) Lowers Serum MMA Levels among Vegetarians (2024)
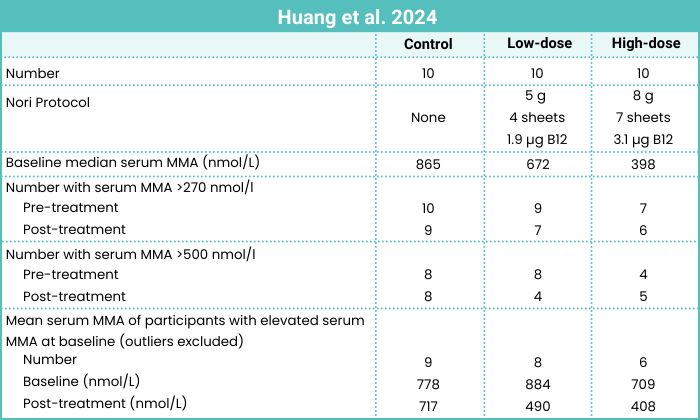
Huang et al. (2024, Taiwan) studied the effect of nori on the B12 status of 24 vegetarians and 6 vegans in a 4-week long trial.
The type of nori used was roasted purple laver (Neopyropia sp., formerly Porphyra sp.). The researchers surveyed the vitamin B12 content of the same brand of nori purchased on different dates and found that the B12 content ranged from 23.1 to 52.8 µg/100 g dry weight. This amount of B12 is similar to that found in purple laver in other studies (see Table 8 above).
Participants were randomized to three groups: control, low-dose nori, and high-dose nori (as described in the table below).
The authors provide a spreadsheet of all the individual participants’ data in their Supplementary Material 3. I sorted the data by treatment group in the Huang 2024 Individuals tab of our spreadsheet, B12 in Plant Foods.
The table below shows that after treatment, serum MMA normalized to ≤ 270 nmol/L in only 4 participants. For such a short trial, reducing MMA to a normal level in most participants might be too much to expect.
Based on a rough correlation between serum MMA levels and clinical symptoms, as discussed in Appendix A. Minimizing Methylmalonic Acid Levels, I did an analysis on Huang et al.’s data using a cutoff for a harmful level of serum MMA of 500 nmol/L. Low-dose nori resulted in 4 of 8 participants reducing their MMA to below 500 nmol/L which seems like an impressive result.
In contrast, high-dose nori resulted in a net increase from 4 to 5 participants with an MMA level >500 nmol/L. There were two participants in the high-dose group whose MMA levels went from healthy to not healthy (306 to 569 and 222 to 819 nmol/L). These participants’ other B12-related parameters didn’t change enough to suggest an obvious decrease in kidney function which is my best guess for what could have caused a substantial increase in MMA levels. The authors didn’t address these two individuals.
There appeared to be no benefit to eating the high-dose amount of nori. This could be due to the saturation of intrinsic factor that occurs with a single dose, although the amount of B12 in the low-dose protocol (1.9 µg) doesn’t seem like enough to saturate intrinsic factor; I would have thought the high-dose protocol would have provided additional benefits.
I consider this study by Huang et al., which used serum MMA, to be easier to interpret than the study by Yamada et al. (1999) below which used urinary MMA. I’m more familiar with serum MMA and it’s correlation with clinical symptoms. The studies also used a different species of nori and are really studying two different foods.
While I’m surprised at how impressive these results are for many of the participants, there are enough inconsistencies that I can’t yet feel comfortable recommending nori as a reliable source of B12 for vegans. I also would feel more comfortable with a study performed using only strict vegans taking no other sources of B12 for the duration of the study.
In summary, it seems possible that dried, purple laver nori (Neopyropia sp., formerly Porphyra sp.) can significantly contribute to improving B12 status for many vegetarians and vegans, but more research is needed before it can be recommended as a sole, reliable source of B12.
Nori (P. tenera) Fails to Lower Urinary MMA Levels (1999)
Yamada et al. (1999, Japan) tested nori (P. tenera) to see if it could reduce urinary methylmalonic acid (uMMA) levels among 10 non-vegetarians. Raw P. tenera was purchased within 48 hours of harvesting and dried P. tenera was purchased from a store. Inactive vs. active B12 was determined by IF assay and confirmed by paper chromatography. The results are shown in Table 9.
| Table 9. Nori’s Impact on Urinary MMA | |||||
|---|---|---|---|---|---|
| Number of Subjects | B12 found to be analogue |
Amount | Duration | uMMA | |
| Dried P. tenera | 6 | 65% | 40 g (20 sheets)A | 6-9 days | increased 77%SS |
| Raw P. tenera | 4 | 27% | 320 g/day A | 3-6 days | increased 5%NS |
|
Source: Yamada, 1999 A. Equivalent amounts of dried food NS. Not statistically significant SS. Statistically significant |
|||||
The results indicate that B12 in raw P. tenera can be changed into harmful inactive B12 analogues by drying, and that dried P. tenera decreases B12 status. Yamada et al. said that although dried nori cannot be used as a B12 source, in small amounts it isn’t harmful. However, they believe that raw nori is an excellent source of active B12.
I disagree with their conclusion that raw nori is an excellent source of active B12. While eating raw nori, the subjects’ uMMA levels increased 5%. While this wasn’t enough a statistically significant increase, it indicates that the raw nori didn’t improve B12 status.
This study was confounded by adding valine (an amino acid that can be converted into MMA when B12 is deficient) to the subjects’ diets in order to increase MMA levels so that a difference could be seen. The valine didn’t appear to do this when given without the nori and no control group was included making the results difficult to interpret.
Coccolithophorid Algae
Coccolithophorid algae (Pleurochrysis carterae) is being used in Japan as a calcium supplement. Miyamoto et al. (Miyamoto, 2001) (2001, Japan) analyzed it for B12 analogue content. Using liquid chromatography, the researchers determined that the B12 analogue was active. They tested it on B12-deficient rats and found that it normalized the rats’ MMA levels. The B12 analogue remained stable for 6 months of storage.
| Table 10. B12 Analogue Content (mcg/30 g) of Coccolithophorid Algae | ||
|---|---|---|
| JapanA | ||
| Assay | Intrinsic Factor | L. delbrueckii |
| Coccolithophorid algae (Pleurochrysis carterae) | 37.6 | 37.6B |
|
A. Miyamoto, 2001 B. Study said the amount was “identical” to that found with Intrinsic Factor. |
||
This same group of researchers later followed up with a second study on coccolithophorid algae (Miyamoto, 2002), but still did not test it to see if it can lower MMA levels in humans.
This algae deserves further attention to see if it can consistently lower MMA levels in humans.
Ulva Fenestrata
Trigo et al. (2024) measured the B12 analogue in the seaweeds, Ulva fenestrata and Palmaria palmata, which are available in Europe. Their results were not peer-reviewed. They found physiologically significant amounts in U. fenestrata but not in P. palmata. They noted that “bioavailability trials are necessary to determine how much vitamin B12 from these products reaches systemic circulation.”
A Case of False Reporting on the Benefit of Seaweed and Fermented Foods
Specker et al. (Specker, 1988) (1988, USA) reported a macrobiotic mother of an infant with a uMMA of 146 µg/mg who modified her diet by increasing her consumption of seaweeds and fermented foods. The infant’s uMMA dropped to 27 µg/mg in 2 months and to 13 µg/mg in 4 months. It was later discovered that this mother had also eaten fish and clam broth which were probably responsible for the improvement rather than the seaweeds and fermented foods (Dagnelie, 1991). Specker et al. stated, “The vegetarian community we worked with believed fermented foods in their diet contained adequate amounts of vitamin B12.” However, on analysis, the fermented foods were shown not to have B12 (Specker, 1988).
Genmai-Saishoku Paradox?
Suzuki (Suzuki, 1995) (1995, Japan) studied 6 vegan children eating a genmai-saishoku (GS) diet, which is based on high intakes of brown rice and contains plenty of sea vegetables, including 2-4 g of nori per day (“dried laver”); as well as hijiki, wakame, and kombu. The foods are organically grown and many are high in cobalt (buckwheat, adzuki beans, kidney beans, shiitake, hijiki). Serum B12 levels of the children are shown in Table 11:
| Table 11. Results of SuzukiB | ||
|---|---|---|
| Age (yrs) | Years Vegan | Serum B12 |
| 7.1 | 4.4 | 520 |
| 7.7 | 4.4 | 720 |
| 8.6A | 8.6 | 480 |
| 8.8A | 8.8 | 300 |
| 12.7 | 10 | 320 |
| 14.6 | 10 | 320 |
| average | 443 (± 164) | |
|
A – Exclusively breast-fed until 6 months old. Mothers had been vegan for 9.6 and 6.5 yrs prior to conception. Both mothers consumed 2 g of nori per day. B – Suzuki, 1995 |
||
None of the many measurements between the vegans and 4 nonvegan controls were significantly different, including serum B12, MCV, and iron indicators. MMA and homocysteine levels were not measured. Some suggestions as to how the vegans got their B12 are:
- From nori or the other seaweeds. The nori was most likely dried.
- Small amounts of B12 from B12 uptake or contamination of plants grown in manure.
- B12 from their mothers’ stores.
These results are both interesting and perplexing. The serum B12 levels are easy to explain as possibly being inactive B12 analogues. But it is particularly impressive that the eight-year-olds were doing well given that their mothers had been vegan for some time, supposedly without B12-fortified foods or supplements. Unfortunately, many vegan children have not had the same positive results, and until more is known about the GS children’s diets, this study should be considered an unsolved mystery.
If these children were my own, I would make sure they started to get at least a modest B12 supplement to ensure their continued good health.
German Whole Foods Vegans Consuming Nori and Mushrooms
In a 2014 study from Germany (Schwarz, 2014), a group of 10 whole foods vegans, who did not take supplements, were found to have MMA levels of almost 400 nmol/l (healthy MMA levels are 270 nmol/l or less). A second group of vegans who supplemented – it’s not clear with how much but it seems to have been at least 2 doses of 1,000 µg/week of B12 on average – had MMA levels of just above 200 nmol/l.
The whole foods-only vegans were given a minimum of 12 g/week of nori and 15 g/week of sun dried mushrooms, which the researchers calculated to contain an average of 3.1 µg/day of vitamin B12. Their MMA levels were measured every 2 months for 8 months and they did not dip much below 350 nmol/l.
The vegans who took supplements were given more B12 than normal (though it’s not clear how much), and their MMA levels steadily decreased to about 150 nmol/l at 6 months, but then back up to 200 nmol/l at 8 months.
This research indicates that at the amounts given, nori and sun dried mushrooms do not improve vitamin B12 status.
Soil and Organic Produce as a B12 Source for Vegans
It’s common in vegan circles to hear that bacteria living in the soil produce vitamin B12 and so if your produce has soil on it, and you don’t wash the produce before eating it, you’ll get B12 from the produce. A related claim is that the modern food supply is more sanitary and, therefore, vegans can’t obtain B12 from it whereas in the past they would have.
What is the evidence for these claims?
B12 Analogue in Soil
There is a one paragraph report often cited in vegan literature for showing that B12 is found in the soil. Robbins et al. (Robbins, 1950) (1950, New York) used Euglena gracilis var. bacillari as a microbiological assay for vitamin B12 “or its physiological equivalent.” A considerable proportion of bacteria and actinomycetes (molds) in the soil were found to synthesize B12 analogues. B12 analogues were also found in the roots of plants (.0002-.01 µg B12/g of fresh material). Some stems had some B12 analogue, but leaves and fruit generally did not. B12 analogue was also found in pond water and pond mud. There was no indication in the report as to how many different soils were tested, but the impression was that it was all in one local area. We don’t know if these B12 analogues were active for humans.
Iranian Villagers
Herbert (Herbert, 1988) reported a group of “vegan” Iranians growing plants in night soil (human manure). The vegetables were eaten without being carefully washed and the amount of B12 was enough to prevent deficiency. However, for this information, Herbert cites Halsted et al. (1959) who do not mention these Iranians in their paper. Herbert possibly meant to cite a 1960 paper by Halsted et al. (1960) which reported that some Iranian villagers with very little animal product intake (dairy once a week, meat once a month) had normal B12 levels. None had megaloblastic anemia. Their average B12 level was 411 pg/ml which was quite high considering their diet. The authors speculated this could be because their diets, which were very low in protein, allowed for B12-producing bacteria to ascend into the ileum where the B12 could be absorbed. The authors also speculated that because the villagers lived among their farm animals and the yards of the village were littered with manure, they might have picked up enough B12 through contamination.
Soybean Plants Absorb B12
Mozafar & Oertli (Mozafar, 1992) (1992, Switzerland) added cyanocobalamin to the soil of soybean plants in amounts ranging from 10 to 3200 µmol/l. Using an intrinsic factor assay, 12-34% of the B12 was absorbed by the plants. 66-87% of the absorbed vitamin remained in the roots and the rest was transported to the various other parts, mainly the leaves. Mozafar points out that the concentrations of B12 in the soil used in this study were many times higher than the reported vitamin concentration in soil solution (.003 µmol/l) measured by Robbins (Robbins, 1950).
Hydroponic Lettuce Absorbs B12
Bito et al (2013) tested to see whether hydroponically grown lettuce would absorb vitamin B12 if it was injected into the growing medium (Bito, 2013). It did so at a rate of .02% to .03%. Enough B12 was absorbed that two lettuce leaves could meet the RDA of 2.4 µg.
Plants Absorb B12 Analogue When Fertilized with Cow Dung
In light of the above results, Mozafar (Mozafar, 1994) (1994, Switzerland) then studied how the B12 levels in plants are affected by adding cow dung to the soil. An assay using pig intrinsic factor was used to measure the B12 analogue. The study looked at the B12 analogue content of both organically fertilized soil and plants.
Two samples were taken from soil that had been treated with organic fertilizer every 5 years over the previous 16 years. The B12 analogue content in these samples was compared to soil that had only synthetic fertilizer applied. Results are shown in Table 12.
| Table 12. B12 Analogue in SoilB | ||
|---|---|---|
| Sample 1 (µg/kg) |
Sample 2 (µg/kg) |
|
| Synthetically fertilized soil | 9 | 5 |
| Organically fertilized soilA | 14 | 10 |
|
A – Treated with organic fertilizer once every 5 years B – Mozafar, 1994 |
||
Soybean, barley, and spinach plants were then grown in pots of 2.5 kg of soil. 10 g dry cow manure was added to each pot. Plant parts were thoroughly washed to remove any soil before B12 was measured. Table 13 shows the results.
| Table 13. B12 Analogue (ng/g) in PlantsC | ||
|---|---|---|
| Nothing Added to Soil | “Organic” (10 g Dry Cow Manure Added) | |
| Soybeans | 1.6 | 2.9 |
| Barley kernels | 2.6A | 9.1A |
| Spinach | 6.9B | 17.8B |
|
A,B – Statistically significant difference between groups with same letters C – Mozafar, 1994 |
||
Further analysis showed that most or all of the B12 analogue in the plants was unbound. Mozafar concluded that plant uptake of B12 from the soil, especially from soil fertilized with manure, could provide some B12 for humans eating the plants, and may be why some vegans, who do not supplement with B12, do not develop B12 deficiency.
Does this mean that organic foods are a good source of B12? No. These studies show that when B12 analogues are placed in the soil, plants can absorb them.
Mushrooms and B12
A 2012 study from the Watanabe group (Watanabe, 2012) found what they thought was active vitamin B12 in the following mushrooms (per 100 g of dry weight):
- 2.9 – 3.9 µg in black trumpet (Craterellus cornucopioides)
- 1.3 – 2.1 µg in golden chanterelle (Cantharellus cibarius)
- 1.3 µg in parasol (Macrolepiota procera)
- .3 – .4 µg in porcini (Boletus spp.)
- .2 µg in oyster (Pleurotus ostreatus)
- .1 µg in black morels (Morchella conica)
The authors noted that 100 g of dry weight was the equivalent of about 1 kg of fresh mushrooms. They said that a moderate intake of black trumpet or golden chanterelle “may contribute slightly to the prevention of severe B12 deficiency in vegetarians.” They did not know why the mushrooms contained B12 and also did not test the mushrooms in humans to determine their ability to lower MMA levels.
In 2009, a paper was published looking at the B12 analogue content of mushrooms in Australia (Koyyalamudi, 2009). The authors used chromatography and mass spectrometry to determine whether the B12 was an active form, and they believed that it was.
Table 14 shows the B12 analogue content of the batches of each mushroom containing the most B12 and the batches containing the least.
| Table 14. B12 in Mushrooms | |||
|---|---|---|---|
| Button | Cup | Flat | |
| Most | |||
| Cap | 1005 | 567 | 161 |
| Flesh | 233 | 83 | 84 |
| Stalk | 17 | 255 | 465 |
| Peel | 217 | 1015 | 354 |
| Total (ng / 400 g) | 1472 | 1920 | 1064 |
| ng / Cupa | 257.60 | 336.00 | 186.20 |
| mcg / Cup | 0.26 | 0.34 | 0.19 |
| Cups to meet RDA | 9.32 | 7.14 | 12.89 |
| Least | |||
| Cap | 11 | 8 | 17 |
| Flesh | 4 | 7 | 4 |
| Stalk | 11 | 7 | 12 |
| Peel | 36 | 20 | 68 |
| Total (ng / 400 g) | 62 | 42 | 101 |
| ng / Cupa | 10.85 | 7.35 | 17.68 |
| mcg / Cup | 0.01 | 0.01 | 0.02 |
| Cups to meet RDA | 221.20 | 326.53 | 135.79 |
| aAssume 70 g per Cup | |||
Assuming that the B12 is active analogue, it would take anywhere from 7 to 326 cups of mushrooms to meet the RDA.
As for the source of the B12, the authors were not sure, but they said:
“The high concentration of vitamin B12 in peel suggests that it was not synthesized within the mushrooms but was either absorbed directly from the compost or synthesized by bacteria on the mushroom surface. The latter is more likely because mushrooms have no root system to take up the vitamin in the compost as is the case with the uptake of vitamins by root plants from the soil containing fertilizers.”
A 2005 study from Italy found significant amounts of vitamin B12 analogue in mushrooms (La Guardia, 2005). 250 g of P. nebrodensis contained 4.8 µg of vitamin B12. They used an immunoenzymatic assay. From the paper, it appears that the soil did not have organic waste of any kind. It is not clear if the B12 analogue was active.
Conclusion About Organic Produce as a B12 Source for Vegans
The suggestion that humans have ever relied on uncleaned, organic produce for vitamin B12 doesn’t have any reliable evidence at this time.
It’s prudent not to assume that vegans who eat organic produce will be protected from B12 deficiency. Unless organic foods are consistently shown to decrease MMA levels, vegans should not rely on them for vitamin B12.
Finally, since the vegan movement’s aim is to eliminate cows on farms, relying on organic foods for vitamin B12 isn’t a long-term solution for providing vitamin B12 for vegans.
Bibliography
Last updated: November 2024
Albert MJ, Mathan VI, Baker SJ. Vitamin B12 synthesis by human small intestinal bacteria. Nature 1980;283(Feb 21):781-2.
Areekul S, Churdchu K, Pungpapong V. Serum folate, vitamin B12 and vitamin B12 binding protein in vegetarians. J Med Assoc Thai 1988 May;71(5):253-7.
Areekul S, Pattanamatum S, Cheeramakara C, Churdchue K, Nitayapabskoon S, Chongsanguan M. The source and content of vitamin B12 in the tempehs. J Med Assoc Thai 1990 Mar;73(3):152-6.
Bissoli L, Di Francesco V, Ballarin A, Mandragona R, Trespidi R, Brocco G, Caruso B, Bosello O, Zamboni M. Effect of vegetarian diet on homocysteine levels. Ann Nutr Metab. 2002;46(2):73-9.
Dagnelie PC, van Staveren WA, van den Berg H. Vitamin B-12 from algae appears not to be bioavailable. Am J Clin Nutr. 1991;53:695-7.
Halsted JA, Carroll J, Rubert S. Serum and tissue concentration of vitamin B12 in. certain pathologic states. N Engl J Med. 1959;260:575-80.
Helrich K, ed. Official Methods of Analysis, Volume 2: Food Composition; Additives; Natural Contaminants, 15th Edition. Arlington, VA: Association of Official Analytical Chemists, Inc; 1990.
Herbert V, Drivas G. Spirulina and Vitamin B12. JAMA. 1982;248(23):3096-7.
Herbert V. Vitamin B-12: plant sources, requirements, and assay. Am J Clin Nutr. 1988;48:852-8.
Lau KS, Gottleib C, Wasserman LR, Herbert V. Measurement of serum B12 level using radioisotopes dilution and coated charcoal. Blood 1965;26:202-8.
Miyamoto E, Watanabe F, Ebara S, Takenaka S, Takenaka H, Yamaguchi Y, Tanaka N, Inui H, Nakano Y. Characterization of a vitamin B12 compound from unicellular coccolithophorid alga (Pleurochrysis carterae). J Agric Food Chem. 2001 Jul;49(7):3486-9.
Mozafar A, Oertli JJ. Uptake of microbially-produced vitamin (B12) by soybean roots. Plant and Soil. 1992;139:23-30.
Mozafar A. Enrichment of some B-vitamins in plants with application of organic fertilizers. Plant & Soil. 1994;167:305-311.
Poston JM. Leucine 2,3-aminomutase: a cobalamin-dependent enzyme present in bean seedlings. Science. 1977;195:301-302.
Poston JM. Coenzyme B12-dependent enzymes in potatoes: leucine 2,3-aminomutase and methylmalonyl-coa mutase. Phytochemistry. 1978;17:401-402.
Pratt R, Johnson E. Deficiency of vitamin B12 in Chlorella. J Pharm Sci. 1968 Jun;57(6):1040-1.
Robbins WJ, Hervey A, Stebbins ME. Studies on Euglena and vitamin B12. Science 1950(Oct 20):455.
Schneider Z, Stroinski A. Comprehensive B12. New York: Walter de Gruyter, 1987.
Specker BL, Miller D, Norman EJ, Greene H, Hayes KC. Increased urinary methylmalonic acid excretion in breast-fed infants of vegetarian mothers and identification of an acceptable dietary source of vitamin B-12. Am J Clin Nutr 1988 Jan;47(1):89-92.
Suzuki H. Serum vitamin B12 levels in young vegans who eat brown rice. J Nutr Sci Vitaminol 1995;41:587-594.
Van den Berg H, Dagnelie PC, van Staveren WA. Vitamin B12 and Seaweed. Lancet Jan 30, 1988.
Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y. Pseudovitamin B(12) is the predominant cobamide of algal health food, spirulina tablets. J Agric Food Chem. 1999 Nov;47(11):4736-41.
Watanabe F, Takenaka S, Katsura H, Masumder SA, Abe K, Tamura Y, Nakano Y. Dried green and purple lavers (Nori) contain substantial amounts of biologically active vitamin B(12) but less of dietary iodine relative to other edible seaweeds. J Agric Food Chem. 1999 Jun;47(6):2341-3.
Watanabe F, Katsura H, Miyamoto E, Takenaka S, Abe K, Yamasaki Y, Nakano Y. Characterization of vitamin B12 in an edible green laver (Entromopha prolifera). Appl Biol Sci 5:99–107, 1999. Not cited. Could not locate copy.
Watanabe F. Vitamin B12 Sources and Bioavailability. Exp Biol Med 2007;232:1266–1274. (PDF) Not cited.
Yamada S, Shibata Y, Takayama M, Narita Y, Sugawara K, Fukuda M. Content and characteristics of vitamin B12 in some seaweeds. J Nutr Sci Vitaminol (Tokyo). 1996 Dec;42(6):497-505. (Abstract)

24 thoughts on “Vitamin B12 in Plant Foods”
Hi!
I’m a vegan who recently found about you and your work, first and foremost I’d like to thank you the good work you’re doing with this web, I find it really insightful.
Secondly, I’d like to ask wether you looked at the research made on Sea Buckthorn and B12. It seems quite promising. https://www.researchgate.net/publication/283944530_Determination_of_Vitamin_B12_in_Sea_Buckthorn_Hippophaes_rhamnoides
It seems like many actinorrizals may have a symbiotic relationship with the bacteria Frankia, which produce B12. The plants not only absorb it but produce B12 themselves through bacterial activity inside them I believe. There’s even less research on other actinorizas and B12 but they’d be interesting to investigate.
Thirdly, there’s a study that uses digestate (anaerobically decomposed plant matter) as a medium to grow chlorella and got a B12 in the chlorella up to 10 micrograms per gram https://www.plantsci.cam.ac.uk/news/vitamin-b12-containing-algae-boosts-fertiliser-digestate-potential
Lastly, I wanted to ask, given your long-lasting research into the topic of B12 in plants, how far away in time do you see the consolidation of a body of research that is enough for a natural vegan source to be considered a reliable B12 source?
(PD: I take my supplements, I’m just interested for self-sufficient/low-tech community building reasons)
Victor,
Thank you for your kind words and for the links to those papers. I’ve mostly given up on reporting research that detects vitamin B12 in a plant food (or in algae or fungi). It’s just too time-consuming. I still report if the research measures the food’s impact on methylmalonic acid levels.
I’d be surprised if there will ever be a time when I think it’s worthwhile to eat a supplemental food product for B12 rather than to simply take B12 supplements. Supplements are inexpensive and reliable, whereas potential plant sources have uncertain variables. I’d rather take the supplement directly than pay more to run the B12 through a plant. That might not be a perfect description in all cases, but close enough.
Have you looked into borș? A Romanian traditional liquid ingredient used to render soups more sour also well-known for its benefits and sometimes drank by itself as a cure.
It’s made by fermenting wheat bran and it’s been found to contain good quantities of B12.
I don’t look into plant foods that might have B12 until there’s been research published about them. If you find some research, please pass it on.
This 2014 nori study results looks strange, could you maybe review them? HoloTC improved, MMA improved only in low dose group. https://link.springer.com/article/10.1007/s00394-024-03505-9
Mykyta,
I cover it in the section on Nori above: https://veganhealth.org/vitamin-b12/vitamin-b12-plant-foods/#nori
Hello and thank you for a good guide for vegan B12. I’ve found a recent study on the topic that might be of interest. The name is “The Seaweed Ulva Fenestrata is a High Source of Biologically Active Vitamin B12: Impact of Biomass Stabilization and Production of a New Alternative Protein Ingredient” and you can find it here: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4901305
I’m not sure it there needs to be more tests on human subjects before we can assume that this is actually a viable source for vegan B12, but it is surely interesting. Especially since the amounts needed are not to big.
It definitely needs more testing before it can be considered a source of vitamin B12.
This?
https://www.sciencedirect.com/science/article/pii/S0308814625015535
Dalibor,
The results of that study, Sea lettuce (Ulva fenestrata) as a rich source of cobalamin (vitamin B12), are impressive enough for me to add U. fenestrata to this article, which I’ll move toward the top of my to-do list. But it’s not enough to promote sea lettuce as a recommended source of B12. As the authors state in their concluding sentence, “Nevertheless, bioavailability trials are necessary to determine the extent to which vitamin B12 from these products is absorbed and reaches systemic circulation.”
Thank you for point me to this study!
I admit I am not a scientifically minded person. Intuition is more my strength. Somewhere I came across info of B 12 being active in the morning dew. In warmer weather I drink this morning dew as well as rub it on my skin. 64 years old. Vegan for 10 years. Last lab tests at doctor revealed my Vitamin B 12 was good. Any thoughts on this?
Luann,
I can’t explain why your B12 status is still adequate after 10 years of apparently not supplementing or eating fortified foods, but there’s no reason to think it’s because you eat morning dew. I would suggest you start making sure you meet our B12 recommendations before your B12 status begins to deteriorate.
I think you are full of BS.. it almost seems like you are trying to sell b12 supplements, and push a contrived narrative to justify it.
I think its time you update this info. 5 years was a long time ago, and the narrative has shifted, in a way that doesnt not warrant your denials of adequacy in non animal product sources of b12 and other analogues
D,
I updated this page in November of 2024, but forgot to change the Last Updated date at the bottom. However, I don’t update this article unless research comes out that I think is relevant enough to justify it. You don’t provide any research to back up your claims that I’m wrong about anything (or everything?) in this article. If there is research to suggest I’m wrong, I want to know about it.
I receive no money from any supplement company—my only goal here is to make sure vegans have the most accurate information possible. I’ve been a vegan since 1988, I’ve read hundreds of studies on vitamin B12 and vegans, and I take a multivitamin with B12 every day.
Another possibility is that vitamin B12 was produced within their microbiote, and that their food, principally bread, was stimulating B12 production because of a high betaine content.
“The foods with the highest betaine concentration (mg/100 g) were: wheat bran (1339), wheat germ (1241), spinach (645), pretzels (237), shrimp (218) and wheat bread (201).”
https://pubmed.ncbi.nlm.nih.gov/12730414/
Betaine is a stimulator of vitamin B12 overproduction in some bacteria
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC240058/
I’m confused by your assessment. Why would studies conclude that foods like nori are good sources of Vitamin B12 if they aren’t? I have seen plenty of studies. This one even tested the absorption in the intestines https://www.ncbi.nlm.nih.gov/m/pubmed/19256490/
They also say drying the nori did not affect B12 levels, unlike what you concluded.
Kelsey,
> Why would studies conclude that foods like nori are good sources of Vitamin B12 if they aren’t?
I wondered the same thing, but they did. I provide their results in the table—they clearly show that nori didn’t improve vitamin B12 status as shown by uMMA increasing in response to both types of nori.
As we state in the article, “It cannot be emphasized enough that until a particular food, obtained from multiple regions, consistently improves vitamin B12 status (by lowering MMA levels) in humans, it should not be relied upon as a source of vitamin B12.”
If plants absorb B12 in the soil (the hydroponic lettuce example and the manure fertiliser example show this) then night soil (human manure – which contains B12) should also be a mechanism by which we could feasibly produce a non-supplement vegan B12 by veganic means (no-livestock agriculture). Your conclusion that we can’t rely on these things currently is sound, but I’m more interested in the principle, that if we set out to produce plants with B12 commercially (without genetic modification), it could be done.
Chris,
I’m not aware of any way to do produce plants that have absorbed B12 that would be both reliable and more efficient than people taking supplements directly—I think there is an economic barrier that doesn’t make this process likely to happen.
The B12 molecule is too large and too hydrophilic to cross biological membranes unaided. That’s why IF is needed to get it across the gut wall, and TC is needed to get it into the cell. So there’s no way that plants would absorb any B12 in the soil.
Hi again,
I have another question:
You stated:
“This article documents many vegans suffering from B12 deficiency, and it is safe to assume that many of them consumed significant amounts of organic foods.”
I was just wondering which articles you referenced that you would use as the basis for this statement. In other words, which articles did you use to determine that the B-12 deficient vegans had “consumed significant amounts of organic foods”?
Thanks in advance for your response.
Back when the B12 section on VeganHealth.org was one very long, single article, I had written this:
“This article documents many vegans suffering from B12 deficiency, and it is safe to assume that many of them consumed significant amounts of organic foods.”
And that referred to many of the cases in the articles under B12 Status of Vegans:
https://veganhealth.org/vitamin-b12/#status
I don’t recall any direct survey measuring organic produce or food intake and B12 deficiency. Rather, I think it’s safe to assume that with the popularity of organic foods among many of the populations those studies were conducted on, and the high incidence of deficiency among those who weren’t supplementing, it provides evidence that organic foods can’t be relied on to prevent deficiency.
I don’t mean to say that there is no B12 in any organic foods—there very well might be. Rather, I’m saying that many of the populations these surveys were conducted on traditionally have eaten large amounts of organic foods and so your average vegan who eats a decent amount of organic food shouldn’t consider themselves safe and should take the usual steps for preventing B12 deficiency. In my opinion, the burden of proof for whether organic foods can prevent vitamin B12 deficiency should be on those suggesting that it can because that view falls outside of the mainstream scientific opinion, including among most vegan health professionals.
Jack, I agree that vegans should rely on a supplement to be safe. However, your assertion that vegans traditionally eat large amounts of organic food is an assumption. You can’t make a scientific conclusion based on an assumption. I’ve been vegan for 23 years and I rarely eat organic food. I think you’ll find that if vegans eat organic food, it is organic grains or legumes. These kinds of foods, being processed, are less likely to contain active B12 than produce. Organic produce, plant food more likely to contain B12, is typically expensive, and the price is a barrier to many people purchasing it, vegan or not. None of the evidence you have provided disproves the claim that the “modern food supply is more sanitary and, therefore, vegans can’t obtain B12 from it whereas in the past they would have.” In fact, the evidence suggests it was possible. That said, the evidence is not conclusive and the only reliable source of B12 is from a supplement. I don’t think that you’d weaken your argument for vegans taking a B12 supplement in 2019 if you merely acknowledged that there is evidence that humans may have obtained active B12 from untreated water, fermented foods or unwashed organic plant foods in the past.
Adam,
To keep things simple, I’ve changed this paragraph (which has a typo):
Given the number of vegans who’ve suffered from B12 deficiency, and that vegans have traditionally interest in organic foods in the U.S. and U.K., it’s safe to assume that organic foods normally don’t provide B12. Only until organic foods are chosen randomly from markets and grocery stores throughout the country (or world) and are consistently shown to decrease MMA levels will someone not be taking a considerable risk in relying on organic foods for B12.
To this:
It’s prudent not to assume that vegans who eat organic produce will be protected from B12 deficiency. Unless organic foods are consistently shown to decrease MMA levels, vegans should not rely on them for vitamin B12.
You said:
> I don’t think that you’d weaken your argument for vegans taking a B12 supplement in 2019 if you merely acknowledged that there is evidence that humans may have obtained active B12 from untreated water, fermented foods or unwashed organic plant foods in the past.
I’ve reviewed that evidence in this section, so in that respect, I am acknowledging it—but it’s not very persuasive. If I’m missing any (relatively direct) evidence, please let me know.